Ion Conductance-Based Perfusability Assay of Vascular Vessel Models in Microfluidic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Microfluidic Devices
2.3. Vascular Vessel Models
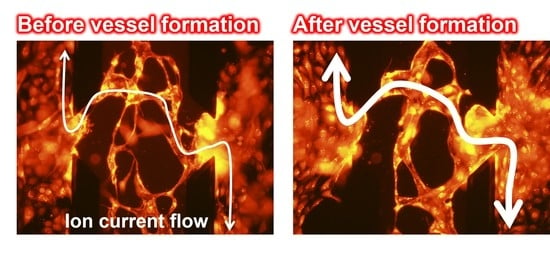
2.4. Ion Conductance Assay for Evaluation of the Perfusability of Vascular Vessel Models
3. Results and Discussion
3.1. Vascular Vessel Models
3.2. Ion Conductance Assay for Evaluating the Perfusability of Vascular Vessel Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henderson, A.R.; Choi, H.; Lee, E. Blood and lymphatic vasculatures on-chip platforms and their applications for organ-specific in vitro modeling. Micromachines 2020, 11, 147. [Google Scholar] [CrossRef] [Green Version]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Ino, K.; Ito, A.; Kumazawa, H.; Kagami, H.; Ueda, M.; Honda, H. Incorporation of capillary-like structures into dermal cell sheets constructed by magnetic force-based tissue engineering. J. Chem. Eng. Jpn. 2007, 40, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Hiramoto, K.; Pai, H.J.; Ino, K.; Nashimoto, Y.; Shiku, H. Electrochemical measurement of respiratory activity for evaluation of fibroblast spheroids containing endothelial cell networks. Electrochim. Acta 2020, 340, 135979. [Google Scholar] [CrossRef]
- Yeon, J.H.; Ryu, H.R.; Chung, M.; Hu, Q.P.; Jeon, N.L. In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip 2012, 12, 2815–2822. [Google Scholar] [CrossRef]
- Patan, S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neuro-Oncol. 2000, 50, 1–15. [Google Scholar] [CrossRef]
- McDonald, D.M.; Choyke, P.L. Imaging of angiogenesis: From microscope to clinic. Nat. Med. 2003, 9, 713–725. [Google Scholar] [CrossRef]
- Utagawa, Y.; Hiramoto, K.; Nashimoto, Y.; Ino, K.; Shiku, H. In vitro electrochemical assays for vascular cells and organs. Electrochem. Sci. Adv. 2021, 2021, e20000189. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for In vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Henry, O.Y.F.; Villenave, R.; Cronce, M.J.; Leineweber, W.D.; Benz, M.A.; Ingber, D.E. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip 2017, 17, 2264–2271. [Google Scholar] [CrossRef]
- Van Dijk, C.G.M.; Brandt, M.M.; Poulis, N.; Anten, J.; van der Moolen, M.; Kramer, L.; Homburg, E.; Louzao-Martinez, L.; Pei, J.Y.; Krebber, M.M.; et al. A new microfluidic model that allows monitoring of complex vascular structures and cell interactions in a 3D biological matrix. Lab Chip 2020, 20, 1827. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.; Park, S.E.; Lu, Q.Z.; Park, K.T.; Cho, M.; Oh, J.M.; Kwon, K.W.; Yi, Y.S.; Song, J.W.; Edelstein, H.I.; et al. Microphysiological engineering of self-assembled and perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano 2019, 13, 7627–7643. [Google Scholar] [CrossRef]
- Yasaki, H.; Yasui, T.; Yanagida, T.; Kaji, N.; Kanai, M.; Nagashima, K.; Kawai, T.; Baba, Y. Substantial expansion of detectable size range in ionic current sensing through pores by using a microfluidic bridge circuit. J. Am. Chem. Soc. 2017, 139, 14137–14142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, F.L.R.; Versloot, R.C.A.; Yakovlieva, L.; Walvoort, M.T.C.; Maglia, G. Protein identification by nanopore peptide profiling. Nat. Commun. 2021, 12, 5795. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Sekiya, Y.; Kawano, R. Hairpin DNA unzipping analysis using a biological nanopore array. Electrochemistry 2016, 84, 338–341. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Piskunen, P.; Nummelin, S.; Liu, Q.; Kostiainen, M.A.; Linko, V. Advanced DNA nanopore technologies. ACS Appl. Bio Mater. 2020, 3, 5606–5619. [Google Scholar] [CrossRef]
- Kawano, R.; Osaki, T.; Sasaki, H.; Takinoue, M.; Yoshizawa, S.; Takeuchi, S. Rapid detection of a cocaine-binding aptamer using biological nanopores on a chip. J. Am. Chem. Soc. 2011, 133, 8474–8477. [Google Scholar] [CrossRef]
- Takai, N.; Shoji, K.; Maki, T.; Kawano, R. Simple fabrication of solid-state nanopores on a carbon film. Micromachines 2021, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Zhou, Y.S.; Miyamoto, T.; Higash, H.; Nakamichi, N.; Takeda, Y.; Kato, Y.; Korchev, Y.; Fukuma, T. High-speed SICM for the visualization of nanoscale dynamic structural changes in hippocampal neurons. Anal. Chem. 2020, 92, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Nashimoto, Y.; Ino, K.; Ida, H.; Imaizumi, T.; Kumatani, A.; Takahashi, Y.; Shiku, H. Micropipet-based navigation in a microvascular model for imaging endothelial cell topography using scanning ion conductance microscopy. Anal. Chem. 2021, 93, 4902–4908. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef]
- Diaz-Santana, A.; Shan, M.; Stroock, A.D. Endothelial cell dynamics during anastomosis in vitro. Integr. Biol. 2015, 7, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.F.; Simmons, C.A. Microfluidic assay for the on-chip electrochemical measurement of cell monolayer permeability. Lab Chip 2019, 19, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.F.; Mohan, M.D.; Young, E.W.K.; Simmons, C.A. Integrated electrochemical measurement of endothelial permeability in a 3D hydrogel-based microfluidic vascular model. Biosens. Bioelectron. 2020, 147, 111757. [Google Scholar] [CrossRef] [PubMed]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef]
- Kanno, Y.; Ino, K.; Abe, H.; Sakamoto, C.; Onodera, T.; Inoue, K.Y.; Suda, A.; Kunikata, R.; Matsudaira, M.; Shiku, H. Electrochemicolor imaging using an LSI-based device for multiplexed cell assays. Anal. Chem. 2017, 89, 12778–12786. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akasaka, R.; Ozawa, M.; Nashimoto, Y.; Ino, K.; Shiku, H. Ion Conductance-Based Perfusability Assay of Vascular Vessel Models in Microfluidic Devices. Micromachines 2021, 12, 1491. https://doi.org/10.3390/mi12121491
Akasaka R, Ozawa M, Nashimoto Y, Ino K, Shiku H. Ion Conductance-Based Perfusability Assay of Vascular Vessel Models in Microfluidic Devices. Micromachines. 2021; 12(12):1491. https://doi.org/10.3390/mi12121491
Chicago/Turabian StyleAkasaka, Rise, Masashi Ozawa, Yuji Nashimoto, Kosuke Ino, and Hitoshi Shiku. 2021. "Ion Conductance-Based Perfusability Assay of Vascular Vessel Models in Microfluidic Devices" Micromachines 12, no. 12: 1491. https://doi.org/10.3390/mi12121491