Anti-Toxoplasma gondii Effects of Lipopeptide Derivatives of Lycosin-I
Abstract
:1. Introduction
2. Results
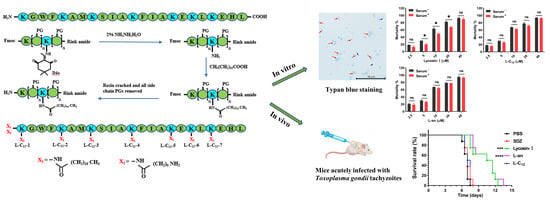
2.1. The Anti-T. gondii Efficacy of Lipopeptides
2.2. L-C12 and L-an Increased the Serum Stability of Anti-T. gondii In Vitro
2.3. L-C12 and L-an Inhibit the Invasion and Proliferation of Tachyzoites into Host Cells
2.4. The Efficacy of Lipopeptides on Anti-T. gondii In Vivo
2.5. The Expression of Inflammatory Factors in Mice
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals, Cells and Parasites
5.2. Lipopeptide Synthesis
5.3. Lipopeptide Screening
5.4. Serum Stability Assay
5.5. Cell Viability Assay
5.6. Invasion Assay
5.7. Intracellular Proliferation Assay
5.8. Plaque Assay
5.9. Survival Assay
5.10. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
5.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T. gondii | Toxoplasma gondii |
| AMPs | antimicrobial peptides |
| CPP | cell penetrating peptide |
| SPF | specific pathogen-free |
| KM mice | Kunming mice |
| HFFs | human foreskin fibroblasts |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| SPPS | solid phase peptide synthesis |
| RP-HPLC | reversed phase-high performance liquid chromatography |
| SDZ | sulfadiazine |
| PYR | pyrimethamine |
| PBS | phosphate-buffered saline |
| MOI | multiplicity of infection |
| PV | parasitophorous vacuole |
| qRT-PCR | quantitative real-time PCR |
| PVM | parasitophorous vacuole membrane |
| IL-12 | interleukin-12 |
| NK cell | natural killer cell |
| IFN-γ | interferon gamma |
| TNF | tumor necrosis factor |
| NO | nitric oxide |
| TGF | transforming growth factor |
References
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Hatam-Nahavandi, K.; Calero-Bernal, R.; Rahimi, M.T.; Pagheh, A.S.; Zarean, M.; Dezhkam, A.; Ahmadpour, E. Toxoplasma gondii infection in domestic and wild felids as public health concerns: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 9509. [Google Scholar] [CrossRef]
- Pittman, K.J.; Knoll, L.J. Long-Term Relationships: The Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiol. Mol. Biol. Rev. 2015, 79, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Flegr, J.; Escudero, D.Q. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects—An explorative cross-sectional study. Parasitology 2016, 143, 1974–1989. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-D.; Liu, H.-H.; Ma, Z.-X.; Ma, H.-Y.; Li, Z.-Y.; Yang, Z.-B.; Zhu, X.-Q.; Xu, B.; Wei, F.; Liu, Q. Toxoplasma gondii Infection in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Front. Microbiol. 2017, 8, 389. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Lyu, C.; Zhao, J.; Shen, B. Sixty Years (1957–2017) of Research on Toxoplasmosis in China-An Overview. Front. Microbiol. 2017, 8, 1825. [Google Scholar] [CrossRef] [Green Version]
- Eyles, D.E.; Coleman, N. Synergistic effect of sulfadiazine and daraprim against experimental toxoplasmosis in the mouse. Antibiot. Chemother. 1953, 3, 483–490. [Google Scholar]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef] [Green Version]
- Antczak, M.; Dzitko, K.; Długońska, H. Human toxoplasmosis–Searching for novel chemotherapeutics. Biomed. Pharmacother. 2016, 82, 677–684. [Google Scholar] [CrossRef]
- Sanfelice, R.A.; da Silva, S.S.; Bosqui, L.R.; Miranda-Sapla, M.M.; Barbosa, B.F.; Silva, R.J.; Ferro, E.A.V.; Panagio, L.A.; Navarro, I.T.; Bordignon, J.; et al. Pravastatin and simvastatin inhibit the adhesion, replication and proliferation of Toxoplasma gondii (RH strain) in HeLa cells. Acta Trop. 2017, 167, 208–215. [Google Scholar] [CrossRef]
- Barbosa, B.F.; Gomes, A.O.; Ferro, E.A.V.; Napolitano, D.R.; Mineo, J.R.; Silva, N.M. Enrofloxacin is able to control Toxoplasma gondii infection in both in vitro and in vivo experimental models. Veter. Parasitol. 2012, 187, 44–52. [Google Scholar] [CrossRef]
- Liu, S.; Wu, M.; Hua, Q.; Lu, D.; Tian, Y.; Yu, H.; Cheng, L.; Chen, Y.; Cao, J.; Hu, X.; et al. Two old drugs, NVP-AEW541 and GSK-J4, repurposed against the Toxoplasma gondii RH strain. Parasites Vectors 2020, 13, 242. [Google Scholar] [CrossRef]
- Abugri, D.A.; Witola, W.H.; Jaynes, J.M.; Toufic, N. In vitro activity of Sorghum bicolor extracts, 3-deoxyanthocyanidins, against Toxoplasma gondii. Exp. Parasitol. 2016, 164, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Huang, B.; Chen, J.; Huang, S.; Zheng, H.; Lun, Z.-R.; Shen, J.; Wang, Y.; Lu, F. In vitro effects of aqueous extracts of Astragalus membranaceus and Scutellaria baicalensis GEORGI on Toxoplasma gondii. Parasitol. Res. 2012, 110, 2221–2227. [Google Scholar] [CrossRef]
- Choi, W.H.; Lee, I.A. Evaluation of Anti-Toxoplasma gondii Effect of Ursolic Acid as a Novel Toxoplasmosis Inhibitor. Pharmaceuticals 2018, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Júnior, E.F.; Menezes, L.F.S.; de Araújo, I.F.S.; Schwartz, E.F. Natural Occurrence in Venomous Arthropods of Antimicrobial Peptides Active against Protozoan Parasites. Toxins 2019, 11, 563. [Google Scholar] [CrossRef] [Green Version]
- Castanheira, L.; de Souza, D.L.N.; Silva, R.J.; Barbosa, B.; Mineo, J.R.; Tudini, K.A.; Rodrigues, R.; Ferro, E.V.; Rodrigues, V.D.M. Insights into anti-parasitism induced by a C-type lectin from Bothrops pauloensis venom on Toxoplasma gondii. Int. J. Biol. Macromol. 2015, 74, 568–574. [Google Scholar] [CrossRef]
- Borges, I.P.; Castanheira, L.E.; Barbosa, B.F.; de Souza, D.L.N.; da Silva, R.J.; Mineo, J.R.; Tudini, K.A.Y.; Rodrigues, R.S.; Ferro, E.A.V.; Rodrigues, V.D.M. Anti-parasitic effect on Toxoplasma gondii induced by BnSP-7, a Lys49-phospholipase A2 homologue from Bothrops pauloensis venom. Toxicon 2016, 119, 84–91. [Google Scholar] [CrossRef]
- Pineda, S.S.; Undheim, E.A.; Rupasinghe, D.B.; Ikonomopoulou, M.P.; King, G.F. Spider venomics: Implications for drug discovery. Future Med. Chem. 2014, 6, 1699–1714. [Google Scholar] [CrossRef]
- De Leon-Nava, M.A.; Romero-Nunez, E.; Luna-Nophal, A.; Bernaldez-Sarabia, J.; Sanchez-Campos, L.N.; Licea-Navarro, A.F.; Morales-Montor, J.; Muniz-Hernandez, S. In Vitro Effect of the Synthetic cal14.1a Conotoxin, Derived from Conus californicus, on the Human Parasite Toxoplasma gondii. Mar. Drugs 2016, 14, 66. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Maeda, H.; Matsuo, T.; Boldbattar, D.; Umemiya-Shirafuji, R.; Kume, A.; Suzuki, H.; Xuan, X.; Tsuji, N.; Fujisaki, K. Parasiticidal activity of Haemaphysalis longicornis longicin P4 peptide against Toxoplasma gondii. Peptides 2012, 34, 242–250. [Google Scholar] [CrossRef]
- Tang, Y.; Hou, S.; Li, X.; Wu, M.; Ma, B.; Wang, Z.; Jiang, J.; Deng, M.; Duan, Z.; Tang, X.; et al. Anti-parasitic effect on Toxoplasma gondii induced by a spider peptide lycosin-I. Exp. Parasitol. 2019, 198, 17–25. [Google Scholar] [CrossRef]
- Hou, S.; Liu, Y.; Tang, Y.; Wu, M.; Guan, J.; Li, X.; Wang, Z.; Jiang, J.; Deng, M.; Duan, Z.; et al. Anti-Toxoplasma gondii effect of two spider venoms in vitro and in vivo. Toxicon 2019, 166, 9–14. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Tang, X.; Wu, M.; Hou, S.; Liu, X.; Li, J.; Deng, M.; Huang, S.; Jiang, L. Anti-Toxoplasma gondii Effects of a Novel Spider Peptide XYP1 In Vitro and In Vivo. Biomedicines 2021, 9, 934. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; De La Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Langenegger, N.; Nentwig, W.; Kuhn-Nentwig, L. Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses. Toxins 2019, 11, 611. [Google Scholar] [CrossRef] [Green Version]
- Bolhassani, A.; Jafarzade, B.S.; Mardani, G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides 2017, 87, 50–63. [Google Scholar] [CrossRef]
- Tan, H.; Ding, X.; Meng, S.; Liu, C.; Wang, H.; Xia, L.; Liu, Z.; Liang, S. Antimicrobial potential of lycosin-I, a cationic and amphiphilic peptide from the venom of the spider Lycosa singorensis. Curr. Mol. Med. 2013, 13, 900–910. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.-J.; Liu, Y.-Y.; Li, H.; Guo, L.-X.; Liu, Z.-H.; Shi, X.-L.; Hu, M. In Vitro Potential of Lycosin-I as an Alternative Antimicrobial Drug for Treatment of Multidrug-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2014, 58, 6999–7002. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Huang, Y.; Xu, J.; Chen, B.; Zhang, P.; Ye, Z.; Liang, S.; Xiao, L.; Liu, Z. Spider Toxin Peptide Lycosin-I Functionalized Gold Nanoparticles for in vivo Tumor Targeting and Therapy. Theranostics 2017, 7, 3168–3178. [Google Scholar] [CrossRef]
- Zhang, P.; Jian, C.; Jian, S.; Zhang, Q.; Sun, X.; Nie, L.; Liu, B.; Li, F.; Li, J.; Liu, M.; et al. Position Effect of Fatty Acid Modification on the Cytotoxicity and Antimetastasis Potential of the Cytotoxic Peptide Lycosin-I. J. Med. Chem. 2019, 62, 11108–11118. [Google Scholar] [CrossRef]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef] [Green Version]
- Henriques, S.T.; Lawrence, N.; Chaousis, S.; Ravipati, A.S.; Cheneval, O.; Benfield, A.H.; Elliott, A.G.; Kavanagh, A.M.; Cooper, M.A.; Chan, L.Y.; et al. Redesigned Spider Peptide with Improved Antimicrobial and Anticancer Properties. ACS Chem. Biol. 2017, 12, 2324–2334. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.; Lu, S.; Chen, Z.; Fan, S.; Chen, D.; Xue, H.; Shi, W.; He, J. Short lipopeptides specifically inhibit the growth of Propionibacterium acnes with a dual antibacterial and anti-inflammatory action. Br. J. Pharmacol. 2019, 176, 2321–2335. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. 2010, 235, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Fu, J.; Sun, L.; Han, Y.; Mao, Q.; Liao, F.; Zheng, X.; Zhu, K. Synthesis and pharmaceutical characterization of site specific mycophenolic acid-modified Xenopus glucagon-like peptide-1 analogs. Medchemcomm 2018, 9, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Mäe, M.; EL Andaloussi, S.; Lundin, P.; Oskolkov, N.; Johansson, H.J.; Guterstam, P.; Langel, U. A stearylated CPP for delivery of splice correcting oligonucleotides using a non-covalent co-incubation strategy. J. Control. Release 2009, 134, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Arukuusk, P.; Pärnaste, L.; Hällbrink, M.; Langel, Ü. PepFects and NickFects for the Intracellular Delivery of Nucleic Acids. Methods Mol. Biol. 2015, 1324, 303–315. [Google Scholar] [CrossRef]
- Lehto, T.; Vasconcelos, L.; Margus, H.; Figueroa, R.; Pooga, M.; Hällbrink, M.; Langel, U. Saturated Fatty Acid Analogues of Cell-Penetrating Peptide PepFect14: Role of Fatty Acid Modification in Complexation and Delivery of Splice-Correcting Oligonucleotides. Bioconjugate Chem. 2017, 28, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Kurtzhals, P.; Havelund, S.; Jonassen, I.; Markussen, J. Effect of Fatty Acids and Selected Drugs on the Albumin Binding of a Long-Acting, Acylated Insulin Analogue. J. Pharm. Sci. 1997, 86, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Liu, T.; Gou, S.; He, Y.; Zhu, N.; Zhu, Y.; Wang, L.; Liu, H.; Zhang, Y.; Yao, J.; et al. Design and synthesis of new N-terminal fatty acid modified-antimicrobial peptide analogues with potent in vitro biological activity. Eur. J. Med. Chem. 2019, 182, 111636. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Qiu, Q.; Ma, K.; Wang, X.; Huang, W.; Qian, H. Aliphatic acid-conjugated antimicrobial peptides--potential agents with anti-tumor, multidrug resistance-reversing activity and enhanced stability. Org. Biomol. Chem. 2015, 13, 7673–7680. [Google Scholar] [CrossRef]
- Jian, C.; Zhang, P.; Ma, J.; Jian, S.; Zhang, Q.; Liu, B.; Liang, S.; Liu, M.; Zeng, Y.; Liu, Z. The Roles of Fatty-Acid Modification in the Activity of the Anticancer Peptide R-Lycosin-I. Mol. Pharm. 2018, 15, 4612–4620. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.G.; Remington, J.S. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 2008, 47, 554–566. [Google Scholar] [CrossRef] [Green Version]
- Paquet, C.; Yudin, M.H. No. 285-Toxoplasmosis in Pregnancy: Prevention, Screening, and Treatment. J. Obstet. Gynaecol. Can. 2018, 40, e687–e693. [Google Scholar] [CrossRef]
- Ardabili, S.; Kohl, J.; Gül, G.; Hodel, M. What obstetricians should be aware of: Serious side effects of antibiotic toxoplasmosis treatment in pregnancy. BMJ Case Rep. 2021, 14, e240809. [Google Scholar] [CrossRef]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R&D 2017, 17, 523–544. [Google Scholar] [CrossRef] [Green Version]
- Borkowski, P.K.; Brydak-Godowska, J.; Basiak, W.; Olszyńska-Krowicka, M.; Rabczenko, D. Adverse Reactions in Antifolate-Treated Toxoplasmic Retinochoroiditis. Adv. Exp. Med. Biol. 2018, 1108, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Guaraldo, L.; Villar, B.B.D.L.F.; Durão, N.M.G.; Louro, V.C.; Quintana, M.D.S.B.; Curi, A.L.L.; Neves, E.S. Ocular toxoplasmosis: Adverse reactions to treatment in a Brazilian cohort. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Shammaa, A.M.; Powell, T.G.; Benmerzouga, I. Adverse outcomes associated with the treatment of Toxoplasma infections. Sci. Rep. 2021, 11, 1035. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef]
- Doliwa, C.; Escotte-Binet, S.; Aubert, D.; Velard, F.; Schmid, A.; Geers, R.; Villena, I. Induction of sulfadiazine resistance in vitro in Toxoplasma gondii. Exp. Parasitol. 2013, 133, 131–136. [Google Scholar] [CrossRef]
- Meneceur, P.; Bouldouyre, M.A.; Aubert, D.; Villena, I.; Menotti, J.; Sauvage, V.; Garin, J.F.; Derouin, F. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob. Agents Chemother. 2008, 52, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.A.; Reis-Cunha, J.L.; Bartholomeu, D.C.; Vítor, R.W. Genetic Polymorphisms and Phenotypic Profiles of Sulfadiazine-Resistant and Sensitive Toxoplasma gondii Isolates Obtained from Newborns with Congenital Toxoplasmosis in Minas Gerais, Brazil. PLoS ONE 2017, 12, e0170689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.J.; Korcsmaros, T.; Carding, S.R. Mechanisms and pathways of Toxoplasma gondii transepithelial migration. Tissue Barriers 2017, 5, e1273865. [Google Scholar] [CrossRef] [Green Version]
- Sasai, M.; Yamamoto, M. Pathogen Recognition Receptors: Ligands and Signaling Pathways by Toll-Like Receptors. Int. Rev. Immunol. 2013, 32, 116–133. [Google Scholar] [CrossRef]
- Quinn, S.R.; O’Neill, L.A. A trio of microRNAs that control Toll-like receptor signalling. Int. Immunol. 2011, 23, 421–425. [Google Scholar] [CrossRef] [Green Version]
- Talevich, E.; Kannan, N. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evol. Biol. 2013, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Behnke, M.S.; Fentress, S.J.; Mashayekhi, M.; Li, L.X.; Taylor, G.A.; Sibley, L.D. The Polymorphic Pseudokinase ROP5 Controls Virulence in Toxoplasma gondii by Regulating the Active Kinase ROP18. PLoS Pathog. 2012, 8, e1002992. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Wernimont, A.; Tang, K.; Taylor, S.; Lunin, V.; Schapira, M.; Fentress, S.; Hui, R.; Sibley, L.D. Novel structural and regulatory features of rhoptry secretory kinases in Toxoplasma gondii. EMBO J. 2009, 28, 969–979. [Google Scholar] [CrossRef] [Green Version]
- Martins-Duarte, S.; de Souza, W.; Vommaro, R.C. Toxoplasma gondii: The effect of fluconazole combined with sulfadiazine and pyrimethamine against acute toxoplasmosis in murine model. Exp. Parasitol. 2013, 133, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Carruthers, V.B. Host cell invasion by the opportunistic pathogen Toxoplasma gondii. Acta Trop. 2002, 81, 111–122. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Wysocka, M.; Hayashi, S.; Denkers, E.Y.; Hieny, S.; Caspar, P.; Trinchieri, G.; Sher, A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1994, 153, 2533–2543. [Google Scholar] [CrossRef]
- Scharton-Kersten, T.; Denkers, E.Y.; Gazzinelli, R.; Sher, A. Role of IL 12 in induction of cell-mediated immunity to Toxoplasma gondii. Res. Immunol. 1995, 146, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.B.; Jensen, K.D.; Saeij, J.P. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 2011, 27, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.M.; Boulter, N.R.; Ikin, R.J.; Smith, N.C. The immunobiology of the innate response to Toxoplasma gondii. Int. J. Parasitol. 2009, 39, 23–39. [Google Scholar] [CrossRef]
- Buzoni-Gatel, D.; Schulthess, J.; Menard, L.C.; Kasper, L.H. Mucosal defences against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell. Microbiol. 2006, 8, 535–544. [Google Scholar] [CrossRef]
- Lang, C.; Groß, U.; Lüder, C.G.K. Subversion of innate and adaptive immune responses by Toxoplasma Gondii. Parasitol. Res. 2007, 100, 191–203. [Google Scholar] [CrossRef]
- Wille, U.; Villegas, E.N.; Striepen, B.; Roos, D.S.; Hunter, C.A. Interleukin-10 does not contribute to the pathogenesis of a virulent strain of Toxoplasma gondii. Parasite Immunol. 2001, 23, 291–296. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, G.; Silva, R.J.; Milián, I.C.B.; Rosini, A.M.; de Araújo, T.E.; Teixeira, S.C.; Oliveira, M.C.; Franco, P.S.; da Silva, C.V.; Mineo, J.R.; et al. Cyclooxygenase (COX)-2 modulates Toxoplasma gondii infection, immune response and lipid droplets formation in human trophoblast cells and villous explants. Sci. Rep. 2021, 11, 12709. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef]





| Time (min) | Flow Rate (mL/min) | A% | B% |
|---|---|---|---|
| 0 | 3 | 80 | 20 |
| 5 | 3 | 80 | 20 |
| 35 | 3 | 10 | 90 |
| 38 | 3 | 10 | 90 |
| 38.1 | 3 | 90 | 10 |
| 41 | 3 | 90 | 10 |
| Name of Primers | Seq of Primers |
|---|---|
| SAG1-F | 5′-CGAGTATGTTTCCGAAGGCAGTGAG-3′ |
| SAG1-R | 5′-GCAGGTGACAACTTGATTGGCAAC-3′ |
| β-Actin (T. gondii)-F | 5′-GCTCTGGCTCCTAGCACCAT-3′ |
| β-Actin (T. gondii)-R | 5′-GCCACCGATCCACACAGAGT-3′ |
| IL-4-F | 5′-TACCAGGAGCCATATCCACGGATG-3′ |
| IL-4-R | 5′-TGTGGTGTTCTTCGTTGCTGTGAG-3′ |
| IL-10-F | 5′-AGAGAAGCATGGCCCAGAAATCAAG-3′ |
| IL-10-R | 5′-CTTCACCTGCTCCACTGCCTTG-3′ |
| IFN-γ-F | 5′-CTGGAGGAACTGGCAAAAGGATGG-3′ |
| IFN-γ-R | 5′-GACGCTTATGTTGTTGCTGATGGC-3′ |
| TNF-α-F | 5′-CACCACGCTCTTCTGTCTACTGAAC-3′ |
| TNF-α-R | 5′-CACACTGTCTTCTTGCCCTCCTAAC-3′ |
| GAPDH (mouse)-F | 5′-TGTTTCCTCGTCCCGTAGA-3′ |
| GAPDH (mouse)-R | 5′-ATCTCCACTTTGCCACTGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, P.; Liu, Y.; Li, J.; Yang, D.; Liu, Z.; Jiang, L. Anti-Toxoplasma gondii Effects of Lipopeptide Derivatives of Lycosin-I. Toxins 2023, 15, 477. https://doi.org/10.3390/toxins15080477
Liu X, Zhang P, Liu Y, Li J, Yang D, Liu Z, Jiang L. Anti-Toxoplasma gondii Effects of Lipopeptide Derivatives of Lycosin-I. Toxins. 2023; 15(8):477. https://doi.org/10.3390/toxins15080477
Chicago/Turabian StyleLiu, Xiaohua, Peng Zhang, Yuan Liu, Jing Li, Dongqian Yang, Zhonghua Liu, and Liping Jiang. 2023. "Anti-Toxoplasma gondii Effects of Lipopeptide Derivatives of Lycosin-I" Toxins 15, no. 8: 477. https://doi.org/10.3390/toxins15080477