Origin of Ciguateric Fish: Quantitative Modelling of the Flow of Ciguatoxin through a Marine Food Chain
Abstract
:1. Introduction
2. Results and Discussion
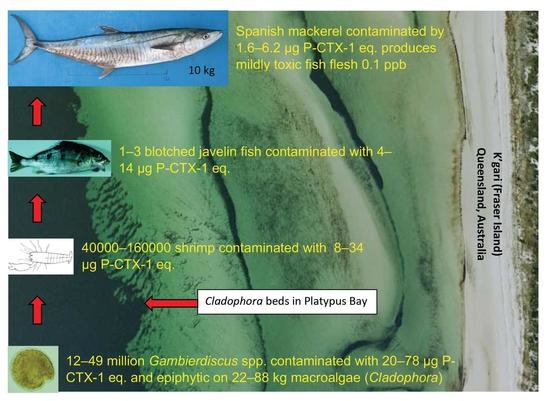
2.1. Trophic Level 1 (Gambierdiscus)
2.2. Trophic Level 2 (Invertebrates)
2.3. Trophic Level 3 (Blotched Javelin Fish)
2.4. The Substrate Supporting Populations of Gambierdiscus in Platypus Bay
3. Implications and Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buckworth, R.C.; Newman, S.J.; Ovenden, J.R.; Lester, R.J.G.; McPherson, G.R. The Stock Structure of Northern and Western Australian Spanish Mackerel; Final Report, Fisheries Research & Development Corporation Project 1988/159; Fishery Report 88; Department of Primary Industry, Fisheries and Mines, Northern Territory Government: Nemarluk, NT, Australia, 2007; Volume i–vi, p. 225. [Google Scholar]
- Gillespie, N.C.; Lewis, R.J.; Pearn, J.H.; Bourke, A.T.C.; Holmes, M.J.; Bourke, J.B.; Shields, W.J. Ciguatera in Australia: Occurrence, clinical features, pathophysiology and management. Med. J. Aust. 1986, 145, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R. Ciguatera in south-eastern Queensland. In Toxic Plants and Animals: A Guide for Australia; Covacevich, J., Davie, P., Pearn, J., Eds.; Queensland Museum: Brisbane, Australia, 1987; pp. 181–187. [Google Scholar]
- Farrell, H.; Murray, S.A.; Zammit, A.; Edwards, A.W. Management of ciguatera risk in eastern Australia. Toxins 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.J.; Venables, B.; Lewis, R.J. Critical review and conceptual and quantitative models for the transfer and depuration of ciguatoxins in fishes. Toxins 2021, 13, 515. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.F.; Langstreth, J.; Buckley, S.M.; Stewart, J. Stock Assessment of Australian East Coast Spanish Mackerel: Predictions of Stock Status and Reference Points; Queensland Government Report; Queensland Government: Brisbane, Australia, 2018; p. 103. Available online: http://era.daf.qld.gov.au/id/eprint/6202/ (accessed on 26 July 2022).
- Tanimoto, M.; Fox, A.R.; O’Neill, M.F.; Langstreth, J.C. Stock Assessment of Australian East Coast Spanish Mackerel (Scomberomorus commerson); Fisheries Queensland, Queensland Department of Agriculture and Fisheries: Brisbane, Australia, 2021; p. 93. Available online: http://era.daf.qld.gov.au/id/eprint/8226/ (accessed on 27 July 2022).
- Holmes, M.J.; Lewis, R.J.; Sellin, M.; Street, R. The origin of ciguatera in Platypus Bay, Australia. Mem. Qld. Mus. 1994, 34, 505–512. [Google Scholar]
- Lewis, R.J.; Holmes, M.J. Origin and transfer of toxins involved in ciguatera. Comp. Biochem. Physiol. 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Lewis, R.J.; Holmes, M.J.; Sellin, M. Invertebrates implicated in the transfer of gambiertoxins to the benthic carnivore Pomadasys maculatus. Mem. Qld. Mus. 1994, 34, 561–564. [Google Scholar]
- Lewis, R.J.; Sellin, M. Multiple ciguatoxins in the flesh of fishes. Toxicon 1992, 30, 915–919. [Google Scholar] [CrossRef]
- Kohli, G.S.; Haslauer, K.; Sarowar, C.; Kretzschmar, A.L.; Boulter, M.; Harwood, D.T.; Laczka, O.; Murray, S.M. Qualitative and quantitative assessment of the presence of ciguatoxin, P-CTX-1b, in Spanish mackerel (Scomberomorus commerson) from waters in New South Wales (Australia). Toxicol. Rep. 2017, 4, 328–334. [Google Scholar] [CrossRef]
- FAO; WHO. Report of the Expert Meeting on Ciguatera Poisoning. Rome, 19–23 November 2018; Food Safety and Quality No. 9; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Fishery and Fishery Products Hazards and Control Guidance. 2022; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition, Table A5–11. Available online: https://www.fda.gov/media/80637/download (accessed on 3 August 2022).
- Sydney Fish Market Seafood Handling Guidelines. 2015. Available online: https://www.sydneyfishmarket.com.au/Portals/0/adam/Content/41UIctIuJECV0p4vxMVS4Q/ButtonLink/Seafood%20Handling%20Guidelines.pdf (accessed on 17 December 2021).
- McPherson, G.R. Age and growth of the narrow-barred Spanish Mackerel (Scomberomorus commerson Lacépède, 1800) in north-eastern Queensland Waters. Aust. J. Mar. Freshw. Res. 1992, 43, 1269–1282. [Google Scholar] [CrossRef]
- Chan, W.H.; Mak, Y.L.; Wu, J.J.; Jin, L.; Sit, W.H.; Lam, J.C.W.; de Mitcheson, Y.S.; Chan, L.L.; Lam, P.K.S.; Murphy, M.B. Spatial distribution of ciguateric fish in the Republic of Kiribati. Chemosphere 2011, 84, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mak, Y.L.; Wai, T.-C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.W.; Chan, L.L.; Lam, P.K.S. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Tech. 2013, 47, 14070–14079. [Google Scholar] [CrossRef] [PubMed]
- Ledreux, A.; Brand, H.; Chinain, M.; Bottein, M.-Y.D. Dynamics of ciguatoxins from Gambierdiscus polynesiensis in the benthic herbivore Mugil cephalus: Trophic transfer implications. Harmful Algae 2014, 39, 165–174. [Google Scholar] [CrossRef]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of ciguatoxins leads to species-specific toxin profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Clausing, R.J.; Losen, B.; Oberhaensli, F.R.; Darius, H.T.; Sibat, M.; Hess, P.; Swarzenski, P.W.; Chinain, M.; Bottein, M.-Y.D. Experimental evidence of dietary ciguatoxin accumulation in an herbivorous coral reef fish. Aquatic Toxicol. 2018, 200, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mak, Y.L.; Chang, Y.-H.; Xiao, C.; Chen, Y.-M.; Shen, J.; Wang, Q.; Ruan, Y.; Lam, P.K.S. Uptake and depuration kinetics of Pacific ciguatoxins in orange-spotted grouper (Epinephelus coioides). Environ. Sci. Technol. 2020, 54, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Leite, I.; Do, P.; Sdiri, K.; Taylor, A.; Viallon, J.; Gharbia, H.B.; Mafra, L.L., Jr.; Swarzenski, P.; Oberhaensli, F.; Darius, H.T.; et al. Experimental evidence of ciguatoxin accumulation and depuration in carnivorous lionfish. Toxins 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.T.; Robertson, A. Depuration Kinetics and Growth Dilution of Caribbean Ciguatoxin in the Omnivore Lagodon rhomboides: Implications for Trophic Transfer and Ciguatera Risk. Toxins 2021, 13, 774. [Google Scholar] [CrossRef]
- Mackie, M.; Gaughan, D.J.; Buckworth, R.C. Stock Assessment of Narrow-Barred Spanish Mackerel (Scomberomorus commerson) in Western Australia. Department of Fisheries: Perth, Australia, FRDC Project No. 1999/151; 2003; p. 242. Available online: https://fish.gov.au/Archived-Reports/Documents/Mackie_et_al_WA_Span_Mack_SA.pdf (accessed on 28 July 2022).
- Darius, H.T.; Paillon, C.; Mou-Tham, G.; Ung, A.; Cruchet, P.; Revel, T.; Viallon, J.; Vigliola, L.; Ponton, D.; Chinain, M. Evaluating age and growth relationship to ciguatoxicity in five coral reef fish species from French Polynesia. Mar. Drugs 2022, 20, 251. [Google Scholar] [CrossRef]
- Sanchez-Henao, A.; García-Àlvarez, N.; Padilla, D.; Ramos-Sosa, M.; Sergent, F.S.; Fernández, A.; Estévez, P.; Gago-Martínez, A.; Diogène, J.; Real, F. Accumulation of C-CTX1 in muscle tissue of goldfish (Carassius auratus) by dietary exposure. Animals 2021, 11, 242. [Google Scholar] [CrossRef]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4830–4836. [Google Scholar] [CrossRef]
- Lucas, R.E.; Lewis, R.J.; Taylor, J.M. Pacific ciguatoxin-1 associated with a large common-source outbreak of ciguatera in east Arnhem Land, Australia. Nat. Toxins 1997, 5, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.R.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Harwood, T.; Smith, K.; Argyle, P.; Munday, R. Production of ciguatoxin and maitotoxin by strains of Gambierdiscus australes, G. pacificus and G. polynesiensis (Dinophyceae) isolated from Rarotonga, Cook Islands. Harmful Algae 2014, 39, 185–190. [Google Scholar] [CrossRef]
- Longo, S.; Sibat, M.; Viallon, J.; Darius, H.T.; Hess, P.; Chinain, M. Intraspecific variability in the toxin production and toxin profiles of in vitro cultures of Gambierdiscus polynesiensis (Dinophyceae) from French Polynesia. Toxins 2019, 11, 735. [Google Scholar] [CrossRef]
- Darius, H.T.; Revel, T.; Viallon, J.; Sibat, M.; Cruchet, P.; Longo, S.; Hardison, D.R.; Holland, W.C.; Tester, P.A.; Litaker, R.W.; et al. Comparative study on the performance of three detection methods for the quantification of Pacific ciguatoxins in French Polynesian strains of Gambierdiscus polynesiensis. Toxins 2022, 20, 348. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.J.; Lewis, R.J. Multiple gambiertoxins (ciguatoxin precursors) from an Australian strain of Gambierdiscus toxicus in culture. In Recent Advances in Toxinology Research; Gopalakrishnakone, P., Tan, C.K., Eds.; National University of Singapore: Singapore, 1992; Volume 2, pp. 520–529. [Google Scholar]
- Satake, M.; Ishibashi, Y.; Legrand, A.M.; Yasumoto, T. Isolation and structure of ciguatoxin-4A, a new ciguatoxin precursor, from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem. 1997, 60, 2103–2105. [Google Scholar] [CrossRef] [PubMed]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics in fish and causative alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef]
- Díaz-Ascenio, L.; Clausing, R.J.; Vandersea, M.; Chamero-Lago, D.; Gómez-Batista, M.; Hernández-Albernas, J.I.; Chomérat, N.; Rojas-Abrahantes, G.; Litaker, R.W.; Tester, P.; et al. Ciguatoxin occurrence in food-web components of a Cuban coral reef ecosystem: Risk-assessment implications. Toxins 2019, 11, 722. [Google Scholar] [CrossRef]
- Lewis, R.J. Ciguatera and Ciguatoxin-Like Substances in Fishes, Especially Scomberomorus commersoni from Southern Queensland. Ph.D. Thesis, University of Queensland, Brisbane, Australia, 1985. [Google Scholar]
- Roué, M.; Darius, H.T.; Ung, A.; Viallon, J.; Sibat, M.; Hess, P.; Amzil, Z.; Chinain, M. Tissue Distribution and elimination of ciguatoxins in Tridacna maxima (Tridacnidae, Bivalvia) fed Gambierdiscus polynesiensis. Toxins 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Roué, M.; Darius, H.T.; Picot, S.; Ung, A.; Viallon, J.; Gaertner-Mazouni, N.; Sibat, M.; Amzil, Z.; Chinain, M. Evidence of the bioaccumulation of ciguatoxins in giant clams (Tridacna maxima) exposed to Gambierdiscus spp. cells. Harmful Algae 2016, 57, 78–87. [Google Scholar] [CrossRef]
- McKay, R.J. Classification of the grunters and javelin-fishes of Australia. Aust. Fish. 1984, 43, 37–40. [Google Scholar]
- Kohli, G.S.; Murray, S.A.; Neilan, B.A.; Rhodes, L.L.; Harwood, T.; Smith, K.F.; Meyer, L.; Capper, A.; Brett, S.; Hallegraeff, G.M. High abundance of the potentially maitotoxic dinoflagellate Gambierdiscus carpenteri in temperate waters of New South Wales, Australia. Harmful Algae 2014, 39, 134–145. [Google Scholar] [CrossRef]
- Gillespie, N.C.; Holmes, M.J.; Burke, J.B.; Doley, J. Distribution and periodicity of Gambierdiscus toxicus in Queensland, Australia. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: Oxford, UK, 1985; pp. 183–188. [Google Scholar]
- Liefer, J.D.; Richlen, M.L.; Smith, T.B.; DeBose, J.L.; Xu, Y.; Anderson, D.M.; Robertson, A. Asynchrony of Gambierdiscus spp. abundance and toxicity in the U.S. Virgin Islands: Implications for monitoring and management of ciguatera. Toxins 2021, 13, 413. [Google Scholar] [CrossRef]
- Gillespie, N.C. Possible origins of ciguatera. In Toxic Plants and Animals, A Guide for Australia; Covacevich, J., Davie, P., Pearn, J., Eds.; Queensland Museum: Brisbane, Australia, 1987; pp. 170–179. [Google Scholar]
- Skinner, M.P.; Lewis, R.J.; Morton, S. Ecology of the ciguatera causing dinoflagellates from the Northern Great Barrier Reef: Changes in community distribution and coastal eutrophication. Mar. Poll. Bull. 2013, 77, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.H.; Coughlan, A.; Hallegraeff, G.; Ajani, P.; Armbrecht, L.; Atikins, N.; Bonham, P.; Brett, S.; Brinkman, R.; Burford, M.; et al. A database of marine phytoplankton abundance, biomass and species composition in Australian waters. Sci. Data 2016, 3, 160043. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.E.; Laczka, O.F.; Harwood, D.T.; Lewis, R.J.; Himaya, S.W.A.; Murray, S.A.; Doblin, M.A. Toxicology of Gambierdiscus spp. (Dinophyceae) from tropical and temperate Australian waters. Mar. Drugs 2018, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Tonge, J.I.; Battey, Y.; Forbes, J.J.; Grant, E.M. Ciguatera poisoning: A report of two outbreaks and a probable fatal case in Queensland. Med. J. Aust. 1967, 2, 1088–1090. [Google Scholar] [CrossRef]
- Yasumoto, T.; Bagnis, R.; Thevenin, S.; Garcon, M. A survey of comparative toxicity in the food chain of ciguatera. Bull. Jap. Soc. Sci. Fish. 1977, 43, 1015–1019. [Google Scholar] [CrossRef]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Tchou Fouc, M.; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar] [CrossRef]
- Chinain, M.; Darius, H.T.; Ung, A.; Fouc, M.T.; Revel, T.; Cruchet, P.; Paullac, S.; Laurent, D. Ciguatera risk management in French Polynesia: The case study of Raivavae Island (Australes Archipelago). Toxicon 2010, 56, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Gaboriau, M.; Ponton, D.; Darius, H.T.; Chinain, M. Ciguatera fish toxicity in French Polynesia: Size does not always matter. Toxicon 2014, 84, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.E. A review of ciguatera, tropical fish poisoning, with a tentative explanation of its cause. Bull. Mar. Sci. 1958, 8, 236–267. [Google Scholar]
- Lewis, R.J.; Sellin, M.; Street, R.; Holmes, M.J.; Gillespie, N.C. Excretion of ciguatoxin from Moray eels (Muraenidae) of the central Pacific. In Proceedings of the Third International Conference on Ciguatera Fish Poisoning, La Parguera, Puerto Rico, 30 April–5 May 1990; Tosteson, T.R., Ed.; Polyscience Publications: Quebec City, QC, Canada, 1992; pp. 131–143. [Google Scholar]
- Chateau-Degat, M.-L.; Chinain, M.; Cerf, N.; Gingras, S.; Hubert, B.; Dewailly, É. Seawater temperature, Gambierdiscus spp. variability and incidence of ciguatera in French Polynesia. Harmful Algae 2005, 4, 1053–1062. [Google Scholar] [CrossRef]
- Parsons, M.L.; Settlemier, C.J.; Bienfang, P.K. A simple model capable of simulating the population dynamics of Gambierdiscus, the benthic dinoflagellate responsible for ciguatera fish poisoning. Harmful Algae 2010, 10, 71–80. [Google Scholar] [CrossRef]

| Spanish Mackerel (kg) | Flesh (kg) Estimated from Equation (1) | Flesh P-CTX-1 (µg) Burden at 0.1 µg/kg | Total P-CTX-1 (µg) Burden in Fish (40% of Toxin Burden) | Total P-CTX-1 (µg) Burden in Fish (10% of Toxin Burden) |
|---|---|---|---|---|
| 10 | 6.2 | 0.62 | 1.6 | 6.2 |
| Modelled Assimilation Efficiency 1 | Trophic level 4: Target P-CTX (µg) Burden in Spanish Mackerel 2 | Trophic Level 3: Required P-CTX (µg) Burden in Blotched Javelin Fish | Trophic Level 2: Required P-CTX (µg) Burden in Shrimps | Trophic Level 1: Required P-CTX (µg) Burden in Gambierdiscus |
|---|---|---|---|---|
| 6% | 1.6–6.2 | 25.9–104 | 431–1,720 | 7,190–28,750 |
| 43% | 1.6–6.2 | 3.6–14.4 | 8.4–33.6 | 19.5–78.1 |
| 100% | 1.6–6.2 | 1.6–6.2 | 1.6–6.2 | 1.6–6.2 |
| Spanish Mackerel (kg) | Trophic Level 2: P-CTX (µg) Burden in Shrimps to Contaminate Trophic Level 3 | Trophic Level 2: Number of Shrimps 2 Required to Contaminate Trophic Level 3 | Trophic Level 1: Gambierdiscus Cells 3 Required to Contaminate Trophic Level 2 | Cladophora Substrate (kg, Wet Weight) to Support 1.2–4.9 × 107 Gambierdiscus 4 |
|---|---|---|---|---|
| 10 | 8.4–33.6 | 40,000–160,000 | 1.2–4.9 × 107 | 22–88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, M.J.; Lewis, R.J. Origin of Ciguateric Fish: Quantitative Modelling of the Flow of Ciguatoxin through a Marine Food Chain. Toxins 2022, 14, 534. https://doi.org/10.3390/toxins14080534
Holmes MJ, Lewis RJ. Origin of Ciguateric Fish: Quantitative Modelling of the Flow of Ciguatoxin through a Marine Food Chain. Toxins. 2022; 14(8):534. https://doi.org/10.3390/toxins14080534
Chicago/Turabian StyleHolmes, Michael J., and Richard J. Lewis. 2022. "Origin of Ciguateric Fish: Quantitative Modelling of the Flow of Ciguatoxin through a Marine Food Chain" Toxins 14, no. 8: 534. https://doi.org/10.3390/toxins14080534