Paralytic Shellfish Toxins of Pyrodinium bahamense (Dinophyceae) in the Southeastern Gulf of Mexico
Abstract
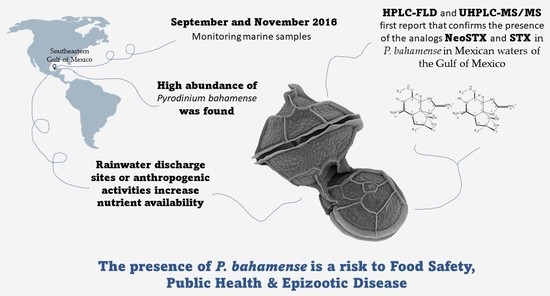
:1. Introduction
2. Results
2.1. Physicochemical Variables
2.2. Identification and Abundance of Pyrodinium bahamense
2.3. Toxicity
2.3.1. Lateral Flow Immunochromatographic Test (Rapid PSP Test)
2.3.2. HPLC-FLD Analysis
2.3.3. UPLC-MS/MS Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Seawater Samples
5.2. Phytoplankton Sampling and Nutrient Determination
5.3. Identification of Pyrodinium bahamense
5.4. Toxicity
5.4.1. Lateral Flow Immunochromatography Test (Rapid PSP Test)
5.4.2. HPLC-FLD Analysis
5.4.3. UPLC-MS/MS
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plate, L. Pyrodinium bahamense n. g., n. sp. die Leucht-Peridninee des “Feuersees” von Nassau, Bahamas. Arch. Protistenk. 1906, 7, 411–429. [Google Scholar]
- Maclean, J.L. Red tide and paralytic shellfish poisoning in Papua New Guinea. Papua New Guinea Agric. J. 1973, 24, 131–138. [Google Scholar]
- Worth, G.K.; Maclean, J.L.; Price, M.J. Paralytic shellfish poisoning in Papua New Guinea, 1972. Pac. Sci. 1975, 29, 1–5. [Google Scholar]
- Hallegraeff, G.; Maclean, J.L. (Eds.) Biology, epidemiology and management of Pyrodinium red tides. In Proceedings of the Management and Training Workshop, ICLARM Conference Proceedings 21, Bandar Seri Begawan, Brunei, 23–30 May 1989; p. 286. [Google Scholar]
- FAO. Marine Biotoxins. In FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; p. 80. [Google Scholar]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef]
- Usup, G.A.; Ahmad, A.A.; Matsuoka, B.K.; Lim, P.T.; Leaw, C.P. Biology, ecology and bloom dynamics of the toxic marine dinoflagellate Pyrodinium bahamense. Harmful Algae 2012, 14, 301–312. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Durán-Riveroll, L.M.; Bustillos-Guzmán, J.J.; Leyva-Valencia, I.; López-Cortés, D.J.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.E.; Ramírez-Rodríguez, D.V. Paralytic toxin producing dinoflagellates in Latin America: Ecology and physiology. Front. Mar. Sci. 2019, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Azanza, R.V.; Taylor, F.J.R. Are Pyrodinium blooms in the Southeast Asian region recurring and spreading? A view at the end of the millennium. AMBIO 2001, 30, 356–364. [Google Scholar] [CrossRef]
- Llewellyn, L.; Negri, A.; Robertson, A. Paralytic shellfish toxins in tropical oceans. Toxin Rev. 2006, 25, 159–196. [Google Scholar] [CrossRef]
- Mertens, K.N.; Wolny, J.; Carbonell-Moore, C.; Bogus, K.; Ellegaard, M.; Limoges, A.; De Vernal, A.; Gurdebeke, P.; Omura, T.; Al-Muftah, A.; et al. Taxonomic re-examination of the toxic armored dinoflagellate Pyrodinium bahamense Plate 1906: Can morphology or LSU sequencing separate P. bahamense var. compressum from var. bahamense? Harmful Algae 2015, 41, 1–24. [Google Scholar] [CrossRef]
- Orellana-Cepeda, E.; Martínez-Romero, E.; Muñoz-Cabrera, L.; López-Ramírez, P.; Cabrera-Mancilla, E.; Ramírez-Camarena, C. Toxicity associated with blooms of Pyrodinium bahamense var. compressum in Southwestern Mexico. In Harmful Algae, Proceedings of the 8th International Conference on Harmful Algae. Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO, Vigo, Spain, 25–29 June 1997; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Vigo, Spain, 1998; p. 60. [Google Scholar]
- Ramírez-Camarena, C.; Rojas-Crisóstomo, R.; Muñoz-Cabrera, L.; Sarniento-Nafate, S.; Juárez-Ruíz, N.O. Mortandad de peces e intoxicaciones humanas en la costa de Chiapas en el 2001. In IX Congreso Nacional de Ciencia y Tecnología del Mar; Nuevo Vallarta: Nayarit, México, 2002; pp. 1–2. [Google Scholar]
- Núñez-Vázquez, E.J.; Bustillos-Guzmán, J.J.; Ramírez-Camarena, C.; Hernández-Sandoval, F. Perfiles cromatográficos de toxinas paralizantes en moluscos bivalvos asociados a Pyrodinium bahamense var. compressum en el Pacífico Sur Mexicano. In Proceedings of the II Taller sobre Florecimientos Algales Nocivos (CICESE-CETMAR), Ensenada, Mexico, 21–23 November 2007; p. 18. [Google Scholar]
- Licea, S.; Navarrete, A.; Rodríguez, R.; Bustillos-Guzmán, J.; Martínez, B.; Ramírez, C. Monitoring a bloom of Pyrodinium bahamense var. compressum in El Salvador and the southern coast of Mexico (November 2005–March 2006). In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; pp. 219–220. [Google Scholar]
- Licea, S.; Navarrete, A.; Castañeda, V.; Bustillos-Guzmán, J.J. Monitoring program for harmful algal blooms in Salvadoran waters: Report of Pyrodinium bahamense from November 2009 to June 2010. In Proceedings of the 14th International Conference on Harmful Algae, International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO 2013; Pagou, K.A., Hallegraeff, G.M., Eds.; IOC-UNESCO: Hersonissos, Greece, 2012. [Google Scholar]
- Amaya, O.; Ruiz, G.; Espinoza, J.; Rivera, W. Saxitoxin analyses with a receptor binding assay (RBA) Suggest PSP Intoxication of sea turtles in El Salvador. Harmful Algal News 2014, 48, 6–7. [Google Scholar]
- Amaya, O.; Dechraoui-Bottein, M.Y.; Leighfield, T.; Ruiz, G. Five years of application of the Receptor Binding Assay (RBA) on seafood products and threatened turtles during outbreaks HABs in El Salvador. In Marine and Fresh-Water Harmful Algae, Proceedings of the 17th International Conference on Harmful Algae, Florianópolis, Brazil, 9–14 October 2016; Proença, L.A.O., Hallegraeff, G.M., Eds.; ISSHA, IOC-UNESCO: Florianópolis, Brasil, 2017; pp. 30–132. [Google Scholar]
- Amaya, O.; Quintanilla, R.; Stacy, B.A.; Dechraoui-Bottein, M.Y.; Flewelling, L.; Hardy, R.; Dueñas, C.; Ruiz, G. Large-scale sea turtle mortality events in El Salvador attributed to paralytic shellfish toxin-producing algae blooms. Front. Mar. Sci. 2018, 5, 411. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; López-Cortés, D.J.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.E. El estado actual del estudio de florecimientos algales nocivos en México. Hidrobiólogica 2011, 21, 381–412. [Google Scholar]
- Emslie, S.D.; Allmon, W.D.; Rich, F.J.; Wrenn, J.H.; Defrance, S.D. Integrated taphonomy of an avian death assemblage in marine sediments from the late Pliocene of Florida. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 124, 107–136. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Hall, S.; Jahannessen, J.N.; White, K.D.; Conrad, S.M.; Abbott, J.P.; Flewellyn, L.J.; Richardson, R.W.; Dickey, R.W.; Jester, E.L.E.; et al. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect. 2006, 114, 1502–1507. [Google Scholar] [CrossRef] [Green Version]
- García-Mendoza, E.; Quijano-Scheggia, S.I.; Olivos-Ortiz, A.; Núñez-Vázquez, E.J.; Pérez-Morales, A. Introducción general. In Florecimientos Algales Nocivos en México; García-Mendoza, E., Quijano-Scheggia, S.I., Olivos-Ortiz, A., Núñez-Vázquez, E.J., Eds.; CICESE: Ensenada, México, 2016; pp. 10–20. [Google Scholar]
- Ochoa, J.L.; Sierra-Beltrán, A.; Alonso-Colmenares, G.; Barrada-Sánchez, H.; Cruz-Villacorta, A.; Núñez-Vázquez, E.J. “Biotoxins in the Pacific Coast of Mexico” in Mycotoxins and Phycotoxins Developments in Chemistry, Toxicology and Food Safety, International Union Purity Analytical Chemistry (IUPAC). In IX IUPAC International Symposium on Mycotoxins and Phycotoxins; Miraglia, M., Van Egmond, H., Brera, C., Gilbert, J., Eds.; Alaken, Inc.: Fort Collins, CO, USA, 1998; pp. 441–448. [Google Scholar]
- Sierra-Beltrán, Á.; Cruz, Á.; Núñez-Vázquez, E.; Del Villar, L.M.; Cerecero, J.; Ochoa, J.L. An overview of the marine food poisoning in Mexico. Toxicon 1998, 36, 1493–1502. [Google Scholar] [CrossRef]
- Núñez-Vázquez, E.J.; Cordero-Tapia, A.; Arnaud, G. Origen e impacto de las Biotoxinas Marinas en la Salud de Tortugas Marinas Salud Pública. In Proceedings of the 1er Encuentro Internacional de Medicina de la Conservación, Vitoria, Spain, 3–7 August 2007. [Google Scholar]
- Núñez-Vázquez, E.J.; Almazán-Becerril, A.; López-Cortés, D.J.; Heredia-Tapia, A.; Hernández-Sandoval, F.E.; Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Gárate-Lizárraga, I.; García-Mendoza, E.; Salinas-Zavala, C.A.; et al. Ciguatera in Mexico (1984–2013). Mar. Drugs 2019, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Morquecho, L. Pyrodinium bahamense One the Most Significant Harmful Dinoflagellate in Mexico. Front. Mar. Sci. 2019, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Rodríguez, R.; Mendoza-Amézquita, E.; Velásquez-López, S.A.; Seim, J.A.; Martínez-Rodríguez, V.M. Florecimientos algales nocivos producidos por Pyrodinium bahamense en Oaxaca, México (2009–2010). Salud Pública México 2015, 57, 343–351. [Google Scholar] [CrossRef]
- Mueren dos Menores Tras Consumir Moluscos Contaminados en Chiapas. Available online: https://www.youtube.com/watch?v=F359KQrArSs (accessed on 29 October 2022).
- Salud Establece Veda Sanitaria por Presencia de Marea Roja en Puerto Madero: Dr. Pepe Cruz. Available online: https://saludchiapas.gob.mx/noticias/post/salud-establece-veda-sanitaria-por-presencia-de-marea-roja-en-puerto-madero-dr-pepe-cruz (accessed on 29 October 2022).
- Gárate-Lizárraga, I.; Pérez-Cruz, B.; Díaz-Ortíz, J.A.; López-Silva, S.; González-Armas, R. Distribución del dinoflagelado Pyrodinium bahamense en la costa pacífica de México. Rev. Latinoam. Ambiente Las Cienc. 2015, 6, 2666–2669. [Google Scholar]
- Balech, E. A revisión of Pyrodinium bahamense Plate (Dinoflagellata). Rev. Palaeobot. Palynol. 1985, 45, 17–34. [Google Scholar] [CrossRef]
- Vargas-Montero, M.; Freer, E. Co-occurrence of different morphotypes of Pyrodinium bahamense during an extensive bloom in Gulf of Nicoya, Costa Rica. In Molluscan Shellfish Safety; Villalba, A., Reguera, B., Romalde, J.L., Beiras, R., Eds.; Xunta de Galicia and IOC-UNESCO: Vigo, Spain, 2003; pp. 211–217. [Google Scholar]
- Garate-Lizárraga, I.; González-Armas, R. Occurrence of Pyrodinium bahamense var. compressum along the southern coast of the Baja California Peninsula. Mar. Pollut. Bull. 2011, 62, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Del Merino-Virgilio, F.C.; Okolodkov, Y.B.; Aguilar-Trujillo, A.C.; Osorio-Moreno, I.; Herrera-Silveira, J.A. Florecimientos algales nocivos en las aguas costeras del norte de Yucatán (2001–2013). In Golfo de México. Contaminación e Impacto Ambiental: Diagnóstico y Tendencias; Botello, A.V., Rendón von Osten, J., Benítez, J.A., Gold-Bouchot, G., Eds.; UAC, UNAM-ICMyL, CINVESTAV-Unidad Mérida: Mérida, Mexico, 2014; pp. 161–180. [Google Scholar]
- Steidinger, K.A.; Tester, L.S.; Taylor, F.J.R. A redescription of Pyrodinium bahamense var. compressa (Bohm) stat. nov. from Pacific red tides. Phycologia 1980, 19, 329–337. [Google Scholar] [CrossRef]
- Gómez-Aguirre, S. First record of Pyrodinium bahamense (Dinoflagellata) in brackish waters of the Mexican Caribbean Coast. Annal. Inst. Biol. UNAM Ser. Zool. 1998, 69, 121–123. [Google Scholar]
- Gómez-Aguirre, S.; Licea, S. Blooms of Pyrodinium bahamense (Dinophyceae) in coastal lagoons of the southern Gulf of Mexico and Mexican Caribbean. In Harmfull Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta Galicia and IOC-UNESCO: Vigo, Spain, 1998; pp. 61–62. [Google Scholar]
- Poot-Delgado, C.A.; Okolodkov, Y.B.; Aké-Castillo, J.A.; Rendón-von Osten, J. Annual cycle of phytoplankton with emphasis on potentially harmful species in oyster beds of Terminos Lagoon, southeastern Gulf of Mexico. Rev. Biol. Mar. Oceanogr. 2015, 50, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Poot-Delgado, C.A. Florecimientos algales nocivos en la costa de Campeche, Golfo de México. Investig. Cienc. Univ. Autónoma Aguascalientes 2016, 68, 91–96. [Google Scholar] [CrossRef]
- Núñez-Vázquez, E.J.; Poot-Delgado, C.A.; Dominguez-Solis, G.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.J. Toxicidad de los botetes silvestres Sphoeroides spp. y Lagocephalus spp. de las costas de Campeche, México. In Proceedings of the Memorias del XX Congreso Nacional de Ciencia y Tecnología del Mar, Cabo San Lucas, Mexico, 1–4 October 2013; pp. 1–5. [Google Scholar]
- Núñez-Vázquez, E.J.; Ramírez-Camarena, C.; Poot-Delgado, C.A.; Almazán-Becerril, A.; Aké-Castillo, J.A.; Pérez-Morales, A.; Hernández-Sandoval, F.E.; Fernández-Herrera, L.J.; Heredia-Tapia, A.; Band-Schmidt, C.J.; et al. Toxinas marinas en el Golfo de México: Orígenes e impactos. In Florecimientos Algales Nocivos en México; García Mendoza, E., Quijano Scheggia, S.I., Olivos-Ortiz, A., Núñez-Vázquez, E.J., Eds.; CICESE: Ensenada, Mexico, 2016; pp. 308–321. [Google Scholar]
- Colmenero, L.M.; Yáñez-Arancibia, A.; Amezcua-Linares, F. Taxonomía, biología y ecología de los tetraodontidos de la Laguna de Términos, Sur del Golfo de México (Pisces: Tetraodontidae). Ann. Inst. Cien. Mar Limnol. 1982, 9, 161–212. [Google Scholar]
- Anon. Official method 959.08. Paralytic shellfish poison. Biological method. Final action. In AOAC Official Methods for Analysis, 18th ed.; Natural Toxins; Truckses, M.W., Ed.; AOAC International: Gaithersburg, MD, USA, 2005; Chapter 49; pp. 79–80. [Google Scholar]
- Anon. Official Method 2005.06 Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Pre-Chromatographic Oxidation and Liquid Chromatography with Fluorescence Detection; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Anon. Official method 2011.02 determination of paralytic shellfish poisoning toxins in mussels, clams, oysters and scallops. In Post-Column Oxidation Method (PCOX). First Action 2011; AOAC International: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Anon. Official Method 2005.06. Report on the Study on the Determination of PSP Toxins in Shellfish Including GTX6 After Hydrolysis Report from the Community Reference Laboratory for Marine Biotoxins; Ministerio de Sanidad y Consumo: Vigo, Spain, 2007. [Google Scholar]
- Turner, A.D.; Hatfield, R.G.; Maskrey, B.H.; Algoet, M.; Lawrence, J.F. Evaluation of the new European Union reference method for paralytic shellfish toxins in shellfish: A review of twelve years regulatory monitoring using pre-column oxidation LC-FLD. Trends Anal. Chem. 2019, 113, 124–139. [Google Scholar] [CrossRef]
- Anon. Official Method 2011.27. Paralytic Shellfish Toxins (PSTs) in Shellfish, Receptor Binding Assay; AOAC International: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography–mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- Turner, A.D.; McNabb, P.S.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single laboratory validation of a multitoxin LC-hydrophilic interaction LC-MS/MS method for quantitation of Paralytic Shellfish Toxins in bivalve shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [CrossRef]
- Turner, A.D.; Tarnovius, S.; Hatfield, R.G.; Teixeira Alves, M.; Broadwater, M.; Van Dolah, F.; Garcia-Mendoza, E.; Medina, D.; Salhi, M.; Goya, A.B.; et al. Application of Six Detection Methods for Analysis of Paralytic Shellfish Toxins in Shellfish from Four Regions within Latin America. Mar. Drugs 2020, 18, 616. [Google Scholar] [CrossRef]
- Núñez-Vázquez, E.J.; Poot-Delgado, C.A.; Turner, A.; Ley-Martínez, T.; Domínguez-Solís, G.; Cahuich-Sánchez, Y.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.J.; Balart, F.E. Presencia de toxinas marinas en peces comerciales de las costas de Campeche. In Memorias IX Foro Científico de Pesca Ribereña; Espino-Barr, E., Gaspar-Dillanes, T., López-González, L., Zúñiga-Flores, M.S., Martínez-Becerril, V.H., Eds.; Instituto Nacional de Pesca y Acuacultura Núm. IX.: Mexico City, Mexico, 2018; pp. 27–28. [Google Scholar]
- Núñez-Vázquez, E.J.; Turner, A.D.; Ramírez-Camarena, C.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.J.; Poot-Delgado, C.A. Profile of paralytic shellfish toxins of Pyrodinium bahamense and first detection of tetrodotoxin in Mexican bivalve mollusks. In Proceedings of the 12th International Conference on Molluscan Shellfish Safety (ICMSS), Ensenada, BC, Mexico, 9–13 September 2019; p. 49. [Google Scholar]
- CNA Ley Federal de Derechos. Disposiciones Aplicables en Materia de Aguas Nacionales 2016; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2016; p. 173. [Google Scholar]
- Osorio-Tafall, B.F. Notas sobre algunos dinoflagelados planctónicos marinos de México con descripción de nuevas especies. Ann. Esc. Nac. Cienc. Biol. 1942, 2, 435–450. [Google Scholar]
- Faust, M.A.; Litaker, W.; Vandersea, M.W.; Kibler, S.R.; Tester, P.A. Dinoflagellate diversity and abundance in two Belizean coral-reef mangrove lagoons: A test of Margalef’s mandala. Atoll Res. Bull. 2005, 534, 104–131. [Google Scholar] [CrossRef]
- Morquecho-Escamilla, L. Morphology of Pyrodinium bahamense Plate (Dinoflagellata) near Isla San José, Gulf of California, Mexico. Harmful Algae 2008, 7, 664–670. [Google Scholar] [CrossRef]
- Poot-Delgado, C.A.; Novelo-Salazar, R.A.; Pérez-Cruz, B. Primer reporte de Pyrodinium bahamense var. bahamense (Gonyaulacales: Goniodomataceae), dinoflagelado tóxico, en la Bahía de Campeche, México. In Proceedings of the Resúmenes del XVI Congreso Nacional de Ciencia y Tecnología del Mar, Colima, Mexico, 5–7 October 2009; p. 2. [Google Scholar]
- Merino-Virgilio, F.D.C.; Aguilar-Trujillo, A.C.; Osorio-Moreno, I.; Okolodkov, Y.B.; Herrera-Silveira, J.A.; Espinosa-Matías, S. Pyrodinium bahamense var. bahamense en el norte de la península de Yucatán. In Proceedings of the Resúmenes del 1er Congreso Nacional de la Sociedad Mexicana para el Estudio de Florecimientos Algales Nocivos, Mazatlán, Sinaloa, 16–18 November 2011; p. 50. [Google Scholar]
- Poot-Delgado, C.A.; Okolodkov, Y.B.; Rendón-von Osten, J. Spatio-temporal variation of harmful planktonic microalgae and cyanobacteria along the central coast of Campeche, southeastern Gulf of Mexico. Bull. Environ. Contam. Toxicol. 2021, 108, 15–23. [Google Scholar] [CrossRef]
- Licea-Duran, S.; Luna-Soria, R. Spatio-temporal variation of phytoplankton on the continental margin in the SW Gulf of Mexico. Rev. Soc. Mex. Hist. Nat. 1999, 49, 83–99. [Google Scholar]
- Gío-Argaez, F.; Machain-Castillo, M.L.; Gaytan-Caballero, A. Los ostrácodos de la zona económica exclusiva de México. Parte I. La bahía de Campeche. Jaina 2002, 13, 1–11. [Google Scholar]
- Poot-Delgado, C.A.; Rosado-García, P.I.; Guzmán-Noz, Y.A. Fitoplancton marino potencialmente nocivo en las aguas costeras de Campeche. In Golfo de México. Contaminación e Impacto Ambiental: Diagnóstico y Tendencias; Botello, A.V., Rendón vonOsten, J., Benítez, J.A., Gold-Bouchot, V., Eds.; UAC, UNAM-ICMyL, CINVESTAV-Unidad Mérida: Mérida, Mexico, 2014; pp. 117–132. [Google Scholar]
- Gracia, A.; Vázquez, G.F.; Enciso-Sánchez, G.; Alexander-Valdés, H.M. Composición y volumen de contaminantes de las descargas costeras al Golfo de México. In Golfo de México. Contaminación e Impacto Ambiental: Diagnóstico y Tendencias; Botello, A.V., Rendón vonOsten, J., Benítez, J.A., Gold-Bouchot, V., Eds.; UAC, UNAM-ICMyL, CINVESTAV-Unidad Mérida: Mérida, Mexico, 2014; pp. 787–816. [Google Scholar]
- Gómez-Figueroa, J.A. Fitoplancton como Bioindicador de la Calidad de Agua: Evaluación de Ecosistemas Acuáticos del Estado de Campeche. Master’s Thesis, Universidad Autónoma de Campeche, Campeche, México, 2020; p. 115. (In Spanish). [Google Scholar]
- Poot-Delgado, C.A.; Okolodkov, Y. Bloom of Cylindrotheca closterium originating from shrimp farming discharges in the SE Gulf of Mexico. Harmful Algae News 2020, 66, 10. [Google Scholar]
- Philips, E.J.; Badylak, S.; Bledsoe, E.; Cichra, M. Factors affecting the distribution of Pyrodinium bahamense var. bahamense in coastal waters of Florida. Mar. Ecol. Prog. Ser. 2006, 322, 99–115. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Gárate-Lizárraga, I. An annotated checklist of dinoflagellates (Dinophyceae) from the Mexican Pacific. Acta Bot. Mex. 2006, 72, 1–154. [Google Scholar] [CrossRef] [Green Version]
- Morquecho-Escamilla, L.; Alonso-Rodríguez, R.; Arreola-Lizarraga, J.A.; Reyes-Salinas, A. Factors associated with moderate blooms of Pyrodinium bahamense in shallow and restricted subtropical lagoons in the Gulf of California. Bot. Mar. 2012, 66, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Morquecho-Escamilla, L.; Alonso-Rodríguez, R.; Martínez-Tecuapacho, G.A. Cyst morphology, germination characteristics, and potential toxicity of Pyrodinium bahamense in the Gulf of California. Bot. Mar. 2014, 54, 303–314. [Google Scholar] [CrossRef]
- Garate-Lizárraga, I.; Pérez-Cruz, B.; Díaz-Ortíz, J.A.; Alarcón-Tacuba, M.A.; Alarcón-Romero, M.A.; Chávez-Almazán, L.A.; García-Barbosa, J.L.; Diego-Balderrama, E. Blooms of Pyrodinium bahamense var. compressum and toxicity in rock oyster at Costa Chica, Guerrero, Mexico. Oceanides 2013, 28, 37–42. [Google Scholar] [CrossRef]
- Garate-Lizárraga, I.; Pérez-Cruz, B.; Díaz-Ortíz, J.A.; Alarcón-Tacuba, M.A.; Chávez-Almazán, M.A.; Alarcón-Romero, M.A.; López-Silva, S.; Bustillos-Guzmán, J.; Licea-Durán, S. Toxicity and paralytic toxin profile in Pyrodinium bahamense var. compressum and violet oyster in Bahía de Acapulco, Guerrero, Mexico. Harmful Algae News 2012, 45, 2–3. [Google Scholar]
- Cusick, K.; Duran, G. sxtA4+ and sxtA4- Genotypes Occur Together within Natural Pyrodinium bahamense Sub-Populations from the Western Atlantic. Microorganisms 2021, 9, 1128. [Google Scholar] [CrossRef]
- Usup, G.; Kulis, D.M.; Anderson, D. Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures. Nat. Toxins 1994, 2, 254–264. [Google Scholar] [CrossRef]
- Hummer, C.; Ritscher, M.; Reinhardt, K.; Lucas, B. Analysis of the characteristic PSP profiles of Pyrodinium bahamense and several strains of Alexandrium by HPLC based on ion-pair chromatographic separation, post-column oxidation, and fluorescence detection. Chromatographia 1997, 45, 312–316. [Google Scholar] [CrossRef]
- Gedaria, A.I.; Luckas, B.; Reinhardt, K.; Azanza, R.V. Growth response and toxin concentration of cultured Pyrodinium bahamense var. compressum to varying salinity and temperature conditions. Toxicon 2007, 50, 519–529. [Google Scholar] [CrossRef]
- Rosales-Loessener, F. The Guatemalan experiences with red tides and paralytic shellfish poisoning. In Biology, Epidemiology, and Management of Pyrodinium Red Tides, Proceedings of the Management and Training Workshop, Bandar Seri Begawan, Brunei Darussalam, 23–30 May 1989; Hallegraeff, G.M., Maclean, J.L., Eds.; WorldFish: Manila, Philippines, 1989; pp. 49–51. [Google Scholar]
- Harada, T.; Oshima, Y.; Kamiya, H.; Yasumoto, T. Confirmation of Paralytic Shellfish Toxins in the Dinoflagellate Pyrodinium bahamense var. compressa and bivalves in Palau. Bull. Japan. Soc. Sci. Fish. 1982, 48, 821–825. [Google Scholar] [CrossRef]
- Rosales-Loessner, F.; De Porras, E.; Y Dix, M.W. Toxic shellfish poisoning in Guatemala. In Red Tides: Biology, Enviromental Science, and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elselvier: New York, NY, USA, 1989; pp. 113–116. [Google Scholar]
- Usup, G.; Cheah, M.Y.; Ng, B.K.; Leaw, C.P.; Ahmad, A. Toxin profile and relative toxicity of three paralytic shellfish poisoning toxin-producing dinoflagellates from Malaysia. Malays. Appl. Biol. 2006, 35, 41–45. [Google Scholar]
- Montojo, U.M.; Sakamoto, S.; Cayme, M.F.; Gatdula, N.C.; Furio, E.F.; Relox, J.R., Jr.; Sato, S.; Fukuyo, Y.; Kodama, M. Remarkable difference in toxin accumulation of paralytic shellfish poisoning toxins among bivalve species exposed to Pyrodinium bahamense var. compressum bloom in Masinloc bay, Philippines. Toxicon 2006, 48, 85–92. [Google Scholar] [CrossRef]
- Banguera-Hinestroza, E.; Eikrem, W.; Mansour, H.; Solberg, I.; Cúrdia, J.; Holtermann, K.; Edvardsen, B.; Kaartvedt, S. Seasonality and toxin production of Pyrodinium bahamense in a Red Sea lagoon. Harmful Algae 2016, 55, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Poot-Delgado, C.A.; Núñez-Vázquez, E.J.; Ruiz-Ibáñez, J.A. Intoxicaciones humanas por consumo de peces botete (Tetraodontidae) en Campeche, México. In Proceedings of the Memorias del XVIII Congreso Nacional de Ciencia y Tecnología del Mar, San Carlos, Mexico, 7–9 September 2011. [Google Scholar]
- Abbott, J.P.; Flewelling, L.J.; Landsberg, J.H. Saxitoxin monitoring in three species of Florida puffer fish. Harmful Algae 2009, 8, 343–348. [Google Scholar] [CrossRef]
- Andersen, P.; Throndsen, J. Estimating cell numbers. In Manual on Harmful Marine Microalgae; Monographs on Oceanographic Methodology no. 11; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO Publishing: Place de Fontenoy, Paris, 2004; pp. 99–130. [Google Scholar]
- Reguera, B.; Méndez, S.; Alonso, R. Quantitative analysis of microalgae: General considerations. In Guide for Designing and Implementing a Plan to Monitor Toxin-Producing Microalgae, 2nd ed.; Reguera, B., Alonso, R., Moreira, A., Méndez, S., Dechraoui-Bottein, M.Y., Eds.; UNESCO: Place de Fontenoy, Paris, 2016; p. 66. [Google Scholar]
- UNEP. Standard Chemical Methods for Marine Environmental Monitoring. Reference Methods for Marine Pollution Studies 50; United Nations Environment Programme: Nairobi, Kenya, 1991; p. 52. [Google Scholar]
- Utermöhl, H. Zurvervolkommung der quantitative. Phytoplankton-Methodik. Verh. Internat. Verein. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Fritz, L.; Triemer, R.E. A rapid simple technique utilizing Calcofluor White M2R for the visualization of dinoflagellate thecal plates. J. Phycol. 1985, 21, 662–664. [Google Scholar] [CrossRef]
- Laycock, M.V.; Donovan, M.A.; Easy, D.J. Sensitivity of lateral flow tests to mixtures of saxitoxins and applications to shellfish and phytoplankton monitoring. Toxicon 2010, 55, 597–605. [Google Scholar] [CrossRef]
- Yu, R.C.; Hummert, C.; Luckas, B.; Qian, P.Y.; Zhou, M.J. Modified HPLC method for analysis of PSP toxins in algae and shellfish from China. Chromatographia 1998, 48, 671–676. [Google Scholar] [CrossRef]
- European Food Safety Authority. Marine biotoxins in shellfish—Saxitoxin group. EFSA J. 2009, 1019, 1–76. [Google Scholar]






| Physicochemical Variables | ||||||||
| T (°C) | Salinity | pH | DO (mg L−1) | |||||
| Stations | September | November | September | November | September | November | September | November |
| S1 | 31 | 27.5 | 37.8 | 24 | 9.29 | 7.06 | 6.2 | 6.9 |
| S2 | 31 | 28.6 | 39.95 | 17 | 9.26 | 6.9 | 6.7 | 4.12 |
| S3 | 31.37 | 28.5 | 40.54 | 21 | 9.05 | 6.5 | 7 | 5.7 |
| S4 | 31.31 | 28.8 | 41.57 | 39 | 9.37 | 7.7 | 7 | 7.45 |
| S5 | 31.79 | 29.4 | 40.01 | 30 | 9.29 | 6.6 | 7.3 | 7.53 |
| S6 | 32.06 | 29.4 | 28.24 | 18 | 9.14 | 7 | 7.5 | 6.53 |
| S7 | 32.8 | 30.1 | 38.42 | 24 | 9.63 | 9 | 7.9 | 4.1 |
| S8 | 32.31 | 38.63 | 39.47 | 35 | 9.29 | 7.3 | 7.7 | 6.79 |
| Mean | 31.71 | 30.12 | 38.25 | 26 | 9.29 | 7.26 | 7.16 | 6.14 |
| SD | 0.65 | 3.52 | 4.21 | 7.96 | 0.17 | 0.79 | 0.55 | 1.37 |
| Nutrients (µmol L−1) | ||||||||
| Nitrites | Nitrates | Ammonium | Orthophosphates | |||||
| Stations | September | November | September | November | September | November | September | November |
| S1 | 0.034 | 0.021 | 0.196 | 0.194 | 0.219 | 0.141 | 0.054 | 0.008 |
| S2 | 0.062 | 0.045 | 0.249 | 0.83 | 0.273 | 0.235 | 0.059 | 0.04 |
| S3 | 0.028 | 0.03 | 0.182 | 0.457 | 0.074 | 0.105 | 0.046 | 0.01 |
| S4 | 0.012 | 0.024 | 0.098 | 0.389 | 0.168 | 0.145 | 0.085 | 0.011 |
| S5 | 0.006 | 0.021 | 0.07 | 0.29 | 0.073 | 0.056 | 0.026 | 0.001 |
| S6 | 0.01 | 0.032 | 0.272 | 0.845 | 0.093 | 0.046 | 0.012 | 0.02 |
| S7 | 0.011 | 0.006 | 0.134 | 0.462 | 0.095 | 0.046 | 0.05 | 0.006 |
| S8 | 0.021 | 0.013 | 0.082 | 0.263 | 0.138 | 0.088 | 0.063 | 0.021 |
| Mean | 0.023 | 0.024 | 0.160 | 0.466 | 0.142 | 0.108 | 0.049 | 0.015 |
| SD | 0.018 | 0.012 | 0.076 | 0.247 | 0.073 | 0.065 | 0.022 | 0.012 |
| * Upper limits established for marine coastal waters | 0.002 | 0.04 | 0.01 | 5 | ||||
| Length (Cell Body): Range or Average, μm | Width: Range or Average, μm | Locality | Reference |
|---|---|---|---|
| 50 | 48 | New Providence Island, the Bahamas | [1] |
| 66 (86 with spines) | 54 | Gulf of Tehuantepec, east coast, and Pacific off El Salvador | [57] |
| 33.8–77.1 (42.5 ± 10.8) | 33.8–67.7 (39.9 ± 7.8) | Tampa Bay, West Florida | [37] |
| 33–47 | 47–53 | Gulf of Nicoya, Costa Rica | [34] |
| 33–77 | 37–67 | Douglas Cay and Twin Cay (coral-reef mangrove lagoons), Belize | [58] |
| 41.9 | 43.8 | Isla San José, Gulf of California | [59] |
| 43 (without the apical horn) | 41.5 | Bahía de Campeche, Gulf of Mexico | [60] |
| 33–47 | 37–52 | Baja California Peninsula, southern coast | [35] |
| 39–59 (48.6 ± 5.0); 48–90 (65.9 ± 11.1) with spines | 40–50 (45.3 ± 2.7) | Marinas along the northern Yucatan Peninsula, Gulf of Mexico | [61] |
| 30–52 | 35–55 | Mexican Pacific | [32] |
| 27.30–81.88 (46.01 ± 9.35) | 22.60–83.34 (48.11 ± 8.97) | Caribbean, Florida | [11] |
| Locality, Year | Profile | Toxin Content (pg STX per Cell) | Reference |
|---|---|---|---|
| Palau, 1980 | Natural: STX, NeoSTX, GTX5 | 1.5–1.4 × 10−4 MU/cell * | [80] |
| Guatemala, 1987 | Natural: STX, NeoSTX, B1, GTX2, GTX3 and GTX4 | ----- | [79,81] |
| Sabah, Malaysia, 1991 | Culture: STX, NeoSTX, GTX5, GTX6 and dcSTX | 0.66–3.98 | [82] |
| Masinloc Bay, Philippines, 2000 and 2002 | Natural: STX, NeoSTX, B1 Culture: STX, NeoSTX, dcSTX, B1 and B2 | 0.66–5.35 0.54–1.33 | [83] |
| Indian River Lagoon, Florida, U.S., 2005 | Natural: STX, B1 and dcSTX Culture: STX and B1 | 3.28 2.02–12.74 | [22] |
| Isla San José, Gulf of California, 2008 | Culture: STX | 0.31 | [72] |
| Lagoon, Red Sea, 2013–2014 | Culture: STX | 2 | [84] |
| Campeche, southeastern Gulf of Mexico, 2016 | Natural: STX and NeoSTX | 6.5 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Vázquez, E.J.; Poot-Delgado, C.A.; Turner, A.D.; Hernández-Sandoval, F.E.; Okolodkov, Y.B.; Fernández-Herrera, L.J.; Bustillos-Guzmán, J.J. Paralytic Shellfish Toxins of Pyrodinium bahamense (Dinophyceae) in the Southeastern Gulf of Mexico. Toxins 2022, 14, 760. https://doi.org/10.3390/toxins14110760
Núñez-Vázquez EJ, Poot-Delgado CA, Turner AD, Hernández-Sandoval FE, Okolodkov YB, Fernández-Herrera LJ, Bustillos-Guzmán JJ. Paralytic Shellfish Toxins of Pyrodinium bahamense (Dinophyceae) in the Southeastern Gulf of Mexico. Toxins. 2022; 14(11):760. https://doi.org/10.3390/toxins14110760
Chicago/Turabian StyleNúñez-Vázquez, Erick J., Carlos A. Poot-Delgado, Andrew D. Turner, Francisco E. Hernández-Sandoval, Yuri B. Okolodkov, Leyberth J. Fernández-Herrera, and José J. Bustillos-Guzmán. 2022. "Paralytic Shellfish Toxins of Pyrodinium bahamense (Dinophyceae) in the Southeastern Gulf of Mexico" Toxins 14, no. 11: 760. https://doi.org/10.3390/toxins14110760