The Inactivation by Curcumin-Mediated Photosensitization of Botrytis cinerea Spores Isolated from Strawberry Fruits
Abstract
:1. Introduction
2. Results
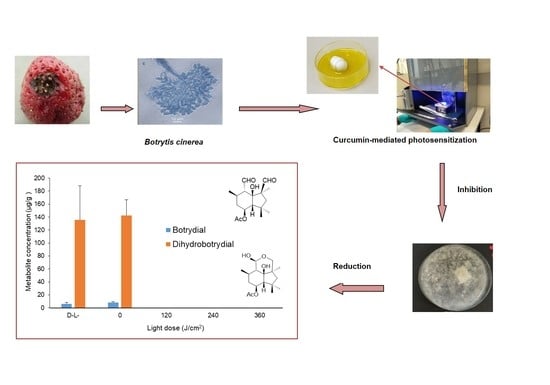
2.1. Extraction of Botrydial and Dihydrobotrydial from B. cinerea Cultures
2.2. HRAM UPLC-MS/MS Analysis of Botrydial and Dihydrobotrydial
2.3. The Effect of Different Curcumin Concentration on Curcumin-Mediated Photosensitization of B. cinerea
2.4. Effect of Curcumin-Mediated Photosensitization on the B. cinerea Spore Using Different Light Doses
2.5. The Growth of B. cinerea over Different Incubation Time After Photosensitization
3. Discussion
3.1. LC-MS/MS Analysis of Metabolites Botrydial and Dihydrobotrydial
3.2. Effect of Curcumin-Mediated Photosensitization on B. cinerea
4. Conclusions
5. Materials and Methods
5.1. Fungal Materials
5.2. Isolation of Botrydial and Dihydrobotrydial Standards
5.3. NMR Analysis of Botrydial and Dihydrobotrydial Standards
5.4. Photosensitizer and Light Source
5.5. Determination of Spore Survival under Differing Photosensitization Conditions
5.6. Determination of Toxin Production under Differing Photosensitization Conditions
5.7. HRAM UPLC-MS/MS Conditions
5.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization Corporate Statistical Database. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/ (accessed on 14 November 2020).
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Penha, C.B.; Bonin, E.; Da Silva, A.F.; Hioka, N.; Zanqueta, É.B.; Nakamura, T.U.; Filho, B.A.D.A.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G. Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT 2017, 76, 198–202. [Google Scholar] [CrossRef]
- Temba, B.A.; Fletcher, M.T.; Fox, G.P.; Harvey, J.; Okoth, S.A.; Sultanbawa, Y.; Temba, B. Curcumin-based photosensitization inactivates Aspergillus flavus and reduces aflatoxin B1 in maize kernels. Food Microbiol. 2019, 82, 82–88. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Tan, B.K.; Hamzah, S.S.; Lin, Y.; Kong, Z.; Zhang, Y.; Zheng, B.; Zeng, S. Photodynamic inactivation of Burkholderia cepacia by curcumin in combination with EDTA. Food Res. Int. 2018, 111, 265–271. [Google Scholar] [CrossRef]
- Foote, C.S. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science 1968, 162, 963–970. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, S.; Netzel, G.; Netzel, M.E.; Mereddy, R.; Phan, A.D.T.; Hong, H.T.; Cozzolino, D.; Sultanbawa, Y. Impact of curcumin-mediated photosensitization on fungal growth, physicochemical properties and nutritional composition in Australian grown strawberry. Food Anal. Methods 2021, 14, 465–472. [Google Scholar] [CrossRef]
- Choquer, M.; Fournier, E.; Kunz, C.; Levis, C.; Pradier, J.-M.; Simon, A.; Viaud, M. Botrytis cinerea virulence factors: New insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 2007, 277, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Fehlhaber, H.-W.; Geipel, R.; Mercker, H.-J.; Tschesche, R.; Welmar, K.; Schönbeck, F. Botrydial, ein sesquiterpen-antibiotikum aus der nährlösung des pilzes Botrytis cinerea. Eur. J. Inorg. Chem. 1974, 107, 1720–1730. [Google Scholar] [CrossRef]
- Durán-Patrón, R.; Hernández-Galán, R.; Rebordinos, L.G.; Cantoral, J.M.; Collado, I.G. Structure-activity relationships of new phytotoxic metabolites with the botryane skeleton from Botrytis cinerea. Tetrahedron 1999, 55, 2389–2400. [Google Scholar] [CrossRef]
- Dalmais, B.; Schumacher, J.; Moraga, J.; Le Pêcheur, P.; Tudzynski, B.; Collado, I.G.; Viaud, M. The Botrytis cinerea phytotoxin botcinic acid requires two polyketide synthases for production and has a redundant role in virulence with botrydial. Mol. Plant Pathol. 2011, 12, 564–579. [Google Scholar] [CrossRef]
- Imada, K.; Tanaka, S.; Ibaraki, Y.; Yoshimura, K.; Ito, S. Antifungal effect of 405-nm light on Botrytis cinerea. Lett. Appl. Microbiol. 2014, 59, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R.; Nyfeler, R. Biosynthesis of the sesquiterpenoids, botrydial and dihydrobotrydial. J. Chem. Soc. Chem. Commun. 1976, 10, 72–73. [Google Scholar] [CrossRef]
- Collado, I.G.; Aleu, J.; Hernandez-Galan, R.; Duran-Patron, R. Botrytis Species: An intriguing source of metabolites with a wide range of biological activities. Structure, chemistry and bioactivity of metabolites isolated from Botrytis species. Curr. Org. Chem. 2000, 4, 1261–1286. [Google Scholar] [CrossRef] [Green Version]
- Niedzwiecki, M.M.; Samant, P.; Walker, D.I.; Tran, V.; Jones, D.P.; Prausnitz, M.R.; Miller, G.W. Human suction blister fluid composition determined using high-resolution metabolomics. Anal. Chem. 2018, 90, 3786–3792. [Google Scholar] [CrossRef] [Green Version]
- El Fellah, S.; Duporté, G.; Sirén, H. Steroid hormones, inorganic ions and botrydial in drinking water. Determination with capillary electrophoresis and liquid chromatography-orbitrap high resolution mass spectrometry. Microchem. J. 2017, 133, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Josewin, S.W.; Kim, M.-J.; Yuk, H.-G. Inactivation of Listeria monocytogenes and Salmonella spp. on cantaloupe rinds by blue light emitting diodes (LEDs). Food Microbiol. 2018, 76, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Temba, B.A.; Fletcher, M.T.; Fox, G.P.; Harvey, J.J.; Sultanbawa, Y. Inactivation of Aspergillus flavus spores by curcumin-mediated photosensitization. Food Control. 2016, 59, 708–713. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J. Photochem. Photobiol. B Biol. 2017, 173, 301–306. [Google Scholar] [CrossRef]
- Araújo, N.C.; De Menezes, R.F.; Carneiro, V.S.M.; Dos Santos-Neto, A.P.; Fontana, C.R.; Bagnato, V.S.; Harvey, C.M.; Gerbi, M.E.M. Photodynamic inactivation of cariogenic pathogens using curcumin as photosensitizer. Photomed. Laser Surg. 2017, 35, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Calzavara-Pinton, P.; Rossi, M.T.; Sala, R.; Venturini, M. Photodynamic antifungal chemotherapy. Photochem. Photobiol. 2012, 88, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-C.; Kang, J.-W.; Shin, J.-I.; Chung, P.-S. Combination treatment with photodynamic therapy and curcumin induces mitochondria-dependent apoptosis in AMC-HN3 cells. Int. J. Oncol. 2012, 41, 2184–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanitá, P.V.; Pavarina, A.C.; Dovigo, L.N.; Ribeiro, A.P.D.; Andrade, M.C.; Mima, E.G.D.O. Curcumin-mediated anti-microbial photodynamic therapy against Candida dubliniensis biofilms. Lasers Med. Sci. 2018, 33, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Lin, C.-F.; Yu, K.-H.; Jheng, C.-P.; Chung, R.; Lee, C.-I. Curcumin reduces amyloid fibrillation of prion protein and decreases reactive oxidative stress. Pathogens 2013, 2, 506–519. [Google Scholar] [CrossRef]
- Wikene, K.O.; Hegge, A.B.; Bruzell, E.; Tønnesen, H.H. Formulation and characterization of lyophilized curcumin solid dispersions for antimicrobial photodynamic therapy (aPDT): Studies on curcumin and curcuminoids LII. Drug Dev. Ind. Pharm. 2014, 41, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Da Frota, M.F.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Bagnato, V.S.; Espir, C.G.; Berbert, F.L.C.V. Photodynamic therapy in root canals contaminated with Enterococcus faecalis using curcumin as photosensitizer. Lasers Med. Sci. 2015, 30, 1867–1872. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.-Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Kielian, T.; Hamblin, M.R. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed. Laser Surg. 2013, 31, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2013, 209, 1963–1971. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yang, Z.; Peng, Y.; Guo, Y.; Yao, M.; Dong, J. Application of 460 nm visible light for the elimination of Candida albicans in vitro and in vivo. Mol. Med. Rep. 2018, 18, 2017–2026. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [Green Version]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Mengiste, T. Plant immunity to necrotrophs. Annu. Rev. Phytopathol. 2012, 50, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; Woltering, E.J.; Staats, M.; Van Kan, J.A.L. Histochemical and genetic analysis of host and non-host interactions of Arabidopsis with three Botrytis species: An important role for cell death control. Mol. Plant Pathol. 2007, 8, 41–54. [Google Scholar] [CrossRef]
- Colmenares, A.J.; Aleu, J.; Durán-Patrón, R.; Collado, I.G.; Hernández-Galán, R. The putative role of botrydial and related metabolites in the infection mechanism of Botrytis cinerea. J. Chem. Ecol. 2002, 28, 997–1005. [Google Scholar] [CrossRef]
- Rossi, F.R.; Gárriz, A.; Marina, M.; Romero, F.M.; Gonzalez, M.E.; Collado, I.G.; Pieckenstain, F.L. The sesquiterpene botrydial produced by Botrytis cinerea induces the hypersensitive response on plant tissues and its action is modulated by salicylic acid and jasmonic acid signaling. Mol. Plant Microbe Interact. 2011, 24, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Durán-Patrón, R.; Cantoral, J.M.; Hernández-Galán, R.; Hanson, J.R.; Collado, I.G. The biodegradation of the phytotoxic metabolite botrydial by its parent organism, Botrytis cinerea. J. Chem. Res. 2004, 2004, 441–443. [Google Scholar] [CrossRef]
- Liñeiro, E.; Macias-Sánchez, A.J.; Espinazo, M.; Cantoral, J.M.; Moraga, J.; Collado, I.G.; Fernández-Acero, F.J. Phenotypic effects and inhibition of botrydial biosynthesis induced by different plant-based elicitors in Botrytis cinerea. Curr. Microbiol. 2017, 75, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, E.F.; Tosati, J.V.; Tikekar, R.V.; Monteiro, A.R.; Nitin, N. Antimicrobial activity of curcumin in combination with light against Escherichia coli O157:H7 and Listeria innocua: Applications for fresh produce sanitation. Postharvest Biol. Technol. 2018, 137, 86–94. [Google Scholar] [CrossRef]
- Tao, R.; Zhang, F.; Tang, Q.-J.; Xu, C.-S.; Ni, Z.-J.; Meng, X.-H. Effects of curcumin-based photodynamic treatment on the storage quality of fresh-cut apples. Food Chem. 2019, 274, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.; Halliday, C.; Alexiou, H.; Ellis, D. Descriptions of Medical Fungi; Newstyle Printing: Adelaide, Australia, 2016; Available online: https://mycology.adelaide.edu.au/docs/fungus3-book.pdf (accessed on 14 November 2020).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]







| Metabolite | Spiking Level µg/g | Detected Conc. (± SD), µg/g | Recovery, (± SD), % | RSD, % |
|---|---|---|---|---|
| Botrydial | 0.11 | 0.07 (0.01) | 66.3 (10.4) | 15.7 |
| 1.02 | 0.84 (0.04) | 81.6 (4.3) | 5.3 | |
| 9.92 | 8.31 (0.44) | 83.8 (4.5) | 5.4 | |
| Average | 77.2 (6.4) | 8.3 | ||
| Dihydrobotrydial | 0.10 | 0.08 (0.01) | 81.9 (7.0) | 8.5 |
| 0.98 | 0.92 (0.06) | 93.5 (6.1) | 6.5 | |
| 9.22 | 6.92 (0.31) | 75.1 (3.4) | 4.5 | |
| Average | 83.5 (5.5) | 6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Yong, K.W.L.; Fernando, W.C.; Carpinelli de Jesus, M.; De Voss, J.J.; Sultanbawa, Y.; Fletcher, M.T. The Inactivation by Curcumin-Mediated Photosensitization of Botrytis cinerea Spores Isolated from Strawberry Fruits. Toxins 2021, 13, 196. https://doi.org/10.3390/toxins13030196
Huang L, Yong KWL, Fernando WC, Carpinelli de Jesus M, De Voss JJ, Sultanbawa Y, Fletcher MT. The Inactivation by Curcumin-Mediated Photosensitization of Botrytis cinerea Spores Isolated from Strawberry Fruits. Toxins. 2021; 13(3):196. https://doi.org/10.3390/toxins13030196
Chicago/Turabian StyleHuang, Li, Ken W. L. Yong, W. Chrishanthi Fernando, Matheus Carpinelli de Jesus, James J. De Voss, Yasmina Sultanbawa, and Mary T. Fletcher. 2021. "The Inactivation by Curcumin-Mediated Photosensitization of Botrytis cinerea Spores Isolated from Strawberry Fruits" Toxins 13, no. 3: 196. https://doi.org/10.3390/toxins13030196