Vitamin D3 Regulates Energy Homeostasis under Short-Term Fasting Condition in Zebrafish (Danio Rerio)
Abstract
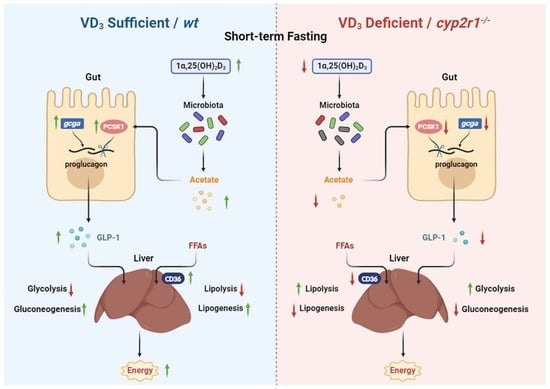
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. Experimental Diets and Feeding Trial
2.3. Antibiotic Treatment
2.4. Sample Collection
2.5. RNA Extraction and Quantitative Real-Time PCR
2.6. Measurement of Glucose, GLP-1 and 1,25(OH)2D3 Contents
2.7. GF Zebrafish Generation and SCFA Treatment
2.8. Calculations and Statistical Analysis
3. Results
3.1. Short-Term Fasting Influences Glucose and Lipid Metabolism in Zebrafish
3.2. 1,25(OH)2D3 Generation in Zebrafish Was Impaired under Short-Term Fasting Condition
3.3. VD3 Regulates Glucose Metabolism in Zebrafish under Short-Term Fasting Condition
3.4. VD3 Regulates Lipid Metabolism in Zebrafish under Short-Term Fasting Condition
3.5. VD3 Promotes the Synthesis and Processing of GLP-1 in the Gut under Short-Term Fasting Condition
3.6. Interaction between VD3 and Gut Microbiota under Short-Term Fasting Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WT | wild type |
| FA | fatty acid |
| FFAs | free fatty acids |
| PC1/3 | prohormone convertase 1/3 |
| PC2 | prohormone convertase 2 |
| GLP-1 | glucagon-like peptide 1 |
| GF | germ-free |
| GZM | gnotobiotic zebrafish medium |
| SCFAs | short-chain fatty acids |
| NaA | sodium acetate |
| NaP | sodium propionate |
| NaB | sodium butyrate |
| hk1 | hexokinase 1 |
| gck | glucokinase |
| pklr | pyruvate kinase L/R |
| pck1 | phosphoenolpyruvate carboxykinase 1 |
| fbp1a | fructose-1,6-bisphosphatase 1a |
| g6p1a.1 | glucose-6-phosphatase catalytic subunit 1a, tandem duplicate 1 |
| ppara | peroxisome proliferator-activated receptor alpha |
| pgc1a | peroxisome proliferator-activated receptor gamma, coactivator 1 alpha |
| pparg | peroxisome proliferator-activated receptor gamma |
| fasn | fatty acid synthase |
| cyp2r1 | cytochrome P450, family 2, subfamily R, polypeptide 1 |
| cyp27b1 | cytochrome P450, family 27, subfamily A, polypeptide 1 |
| cyp24a1 | cytochrome P450, family 24, subfamily A, polypeptide 1 |
| vdra | vitamin D receptor a |
| vdrb | vitamin D receptor b |
| insra | insulin receptor a |
| insrb | insulin receptor b |
| gcgra | glucagon receptor a |
| gcgrb | glucagon receptor b |
| gip | gastric inhibitory polypeptide |
| gipr | gastric inhibitory polypeptide receptor |
| pyya | peptide YY a |
| pyyb | peptide YY b |
| sglt | sodium-glucose cotransporter 1 |
| cd36 | CD36 molecule |
| slc27a2a | solute carrier family 27 member 2a |
| gcga | glucagon a |
| gcgb | glucagon b |
| pcsk1 | proprotein convertase subtilisin/kexin type 1 |
| pcsk2 | proprotein convertase subtilisin/kexin type 2 |
| actb2 | actin, beta 2 |
References
- Wang, T.; Hung, C.C.Y.; Randall, D.J. The Comparative Physiology of Food Deprivation: From Feast to Famine. Annu. Rev. Physiol. 2006, 68, 223–251. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1. [Google Scholar] [CrossRef]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef]
- Gou, N.; Wang, K.; Jin, T.; Yang, B. Effects of Starvation and Refeeding on Growth, Digestion, Nonspecific Immunity and Lipid-Metabolism-Related Genes in Onychostoma macrolepis. Animals 2023, 13, 1168. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Liu, H.; Xie, S. Effects of starvation on glucose and lipid metabolism in gibel carp (Carassius auratus gibelio var. CAS III). Aquaculture 2018, 496, 166–175. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, W.; Kenzior, A.; Olsen, L.; Krishnan, J.; Persons, J.; Medley, K.; Peuß, R.; Wang, Y.; Chen, S.; et al. Enhanced lipogenesis through Pparγ helps cavefish adapt to food scarcity. Curr. Biol. 2022, 32, 2272–2280.e2276. [Google Scholar] [CrossRef] [PubMed]
- Navarro, I.; Gutiérrez, J. Chapter 17 Fasting and starvation. In Biochemistry and Molecular Biology of Fishes; Hochachka, P.W., Mommsen, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 4, pp. 393–434. [Google Scholar]
- Secor, S.M.; Carey, H.V. Integrative Physiology of Fasting. Compr. Physiol. 2016, 6, 773–825. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Metabolic Messengers: Glucagon-like peptide 1. Nat. Metab. 2021, 3, 142–148. [Google Scholar] [CrossRef]
- Plisetskaya, E.M.; Mommsen, T.P. Glucagon and Glucagon-like Peptides in Fishes. Int. Rev. Cytol. 1996, 168, 187–257. [Google Scholar]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-h.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.N.; Luo, J.N.; Harris, D.A.; Aliakbarian, H.; Yao, L.; Paik, D.; Subramaniam, R.; Adhikari, A.A.; Vernon, A.H.; Kiliç, A.; et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe 2021, 29, 408–424.e407. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, D.; Ding, C.Z.; Guo, F.; Wu, L.N.; Huang, F.J.; Liu, Y.L.; Zhao, S.Y.; Xin, Y.; Ma, S.N.; et al. MicroRNA-194: A novel regulator of glucagon-like peptide-1 synthesis in intestinal L cells. Cell Death Dis. 2021, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cheng, D.; Wang, L.; Chen, F.; Chen, H.; Ma, H.; Yang, Y.; Yuan, X. GLP-1 responds to postprandial hyperglycemia by reducing transcription level in grass carp (Ctenopharyngodon idella). Aquac. Rep. 2022, 23, 101045. [Google Scholar] [CrossRef]
- Chivite, M.; Naderi, F.; Conde-Sieira, M.; Soengas, J.L.; Lopez-Patiño, M.A.; Míguez, J.M. Central serotonin participates in the anorexigenic effect of GLP-1 in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2021, 304, 113716. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Aatsinki, S.M.; Elkhwanky, M.S.; Kummu, O.; Karpale, M.; Buler, M.; Viitala, P.; Rinne, V.; Mutikainen, M.; Tavi, P.; Franko, A.; et al. Fasting-Induced Transcription Factors Repress Vitamin D Bioactivation, a Mechanism for Vitamin D Deficiency in Diabetes. Diabetes 2019, 68, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Żychowska, M.; Rola, R.; Borkowska, A.; Tomczyk, M.; Kortas, J.; Anczykowska, K.; Pilis, K.; Kowalski, K.; Pilch, W.; Antosiewicz, J. Fasting and Exercise Induce Changes in Serum Vitamin D Metabolites in Healthy Men. Nutrients 2021, 13, 1963. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Liao, X.; Lan, Y.; Zhang, H.; Jiao, L.; Du, Q.; Han, D.; Ai, Q.; Mai, K.; Wan, M. Vitamin D regulates insulin pathway and glucose metabolism in zebrafish (Danio rerio). FASEB J. 2022, 36, e22330. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Liao, X.; Wang, W.; Lan, Y.; Zhang, H.; Du, Q.; Jiao, L.; Yin, Z.; Ai, Q.; Mai, K.; et al. Vitamin D Regulates Glucose Metabolism in Zebrafish (Danio rerio) by Maintaining Intestinal Homeostasis. J. Nutr. Biochem. 2023, 123, 109473. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty Acid Oxidation in Zebrafish Adipose Tissue Is Promoted by 1α,25(OH)(2)D(3). Cell Rep. 2017, 19, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lan, Y.; Shao, R.; Liu, J.; Liang, S.; Yin, Z.; Gudmundsson, G.H.; Bergman, P.; Wan, M. Vitamin D Enhances Neutrophil Generation and Function in Zebrafish (Danio rerio). J. Innate Immun. 2021, 14, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. A novel, reliable method for repeated blood collection from aquarium fish. Zebrafish 2013, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.N.; Kanther, M.; Semova, I.; Rawls, J.F. Methods for generating and colonizing gnotobiotic zebrafish. Nat. Protoc. 2008, 3, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Coate, K.C.; Kliewer, S.A.; Mangelsdorf, D.J. SnapShot: Hormones of the gastrointestinal tract. Cell 2014, 159, 1478.e1471. [Google Scholar] [CrossRef]
- Liao, X.; Lan, Y.; Wang, W.; Zhang, J.; Shao, R.; Yin, Z.; Gudmundsson, G.H.; Bergman, P.; Mai, K.; Ai, Q.; et al. Vitamin D influences gut microbiota and acetate production in zebrafish (Danio rerio) to promote intestinal immunity against invading pathogens. Gut Microbes 2023, 15, 2187575. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Jones, J.G. Hepatic glucose and lipid metabolism. Diabetologia 2016, 59, 1098–1103. [Google Scholar] [CrossRef]
- Roden, M.; Stingl, H.; Chandramouli, V.; Schumann, W.C.; Hofer, A.; Landau, B.R.; Nowotny, P.; Waldhäusl, W.; Shulman, G.I. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 2000, 49, 701–707. [Google Scholar] [CrossRef]
- Perry Rachel, J.; Camporez, J.-P.G.; Kursawe, R.; Titchenell Paul, M.; Zhang, D.; Perry Curtis, J.; Jurczak Michael, J.; Abudukadier, A.; Han Myoung, S.; Zhang, X.-M.; et al. Hepatic Acetyl CoA Links Adipose Tissue Inflammation to Hepatic Insulin Resistance and Type 2 Diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef]
- van Ginneken, V.; Verhey, E.; Poelmann, R.; Ramakers, R.; van Dijk, K.W.; Ham, L.; Voshol, P.; Havekes, L.; Van Eck, M.; van der Greef, J. Metabolomics (liver and blood profiling) in a mouse model in response to fasting: A study of hepatic steatosis. Biochim. Et. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2007, 1771, 1263–1270. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Yang, G.; Xu, K.; Yin, Y.; Brecchia, G.; Yin, J. CD36 favours fat sensing and transport to govern lipid metabolism. Prog. Lipid Res. 2022, 88, 101193. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef]
- Polakof, S.; Míguez, J.M.; Soengas, J.L. Evidence for a gut-brain axis used by glucagon-like peptide-1 to elicit hyperglycaemia in fish. J. Neuroendocrinol. 2011, 23, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.M.; Mojsov, S. Diversification of the functions of proglucagon and glucagon receptor genes in fish. Gen. Comp. Endocrinol. 2018, 261, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: A systematic review of in vivo studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- Vanherwegen, A.S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- Del Pinto, R.; Ferri, C.; Cominelli, F. Vitamin D Axis in Inflammatory Bowel Diseases: Role, Current Uses and Future Perspectives. Int. J. Mol. Sci. 2017, 18, 2360. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Kumar, V.; Khare, P.; Devi, K.; Kaur, J.; Kumar, V.; Kiran Kondepudi, K.; Chopra, K.; Bishnoi, M. Short-chain fatty acids increase intracellular calcium levels and enhance gut hormone release from STC-1 cells via transient receptor potential Ankyrin1. Fundam. Clin. Pharmacol. 2021, 35, 1004–1017. [Google Scholar] [CrossRef]
- Leung, P.S. The Potential Protective Action of Vitamin D in Hepatic Insulin Resistance and Pancreatic Islet Dysfunction in Type 2 Diabetes Mellitus. Nutrients 2016, 8, 147. [Google Scholar] [CrossRef]
- Viloria, K.; Hewison, M.; Hodson, D.J. Vitamin D binding protein/GC-globulin: A novel regulator of alpha cell function and glucagon secretion. J. Physiol. 2022, 600, 1119–1133. [Google Scholar] [CrossRef]
- Cryer, P.E. The Barrier of Hypoglycemia in Diabetes. Diabetes 2008, 57, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Desouza, C.V.; Bolli, G.B.; Fonseca, V. Hypoglycemia, Diabetes, and Cardiovascular Events. Diabetes Care 2010, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]






| Ingredient (g/kg) | Non-VD3 Diet | VD3 Diet |
|---|---|---|
| Casein | 320 | 320 |
| Gelatin | 80 | 80 |
| Corn starch | 300 | 300 |
| Soybean oil | 70 | 70 |
| Choline chloride | 5 | 5 |
| Monocalcium phosphate | 20 | 20 |
| Carboxymethyl cellulose | 21 | 21 |
| Sodium alginate | 6 | 6 |
| Vitamin premix a (VD3-free) | 10 | 10 |
| Mineral premix b | 30 | 30 |
| Cellulose | 138 | 138 |
| Vitamin D3 (IU/kg) | 0 | 800 |
| Total | 1000 | 1000 |
| Proximate composition (dry matter basis %) | ||
| Moisture | 8.64 | 8.36 |
| Crude protein | 35.7 | 35.7 |
| Crude lipid | 5.11 | 5.12 |
| Ash | 3.65 | 3.63 |
| Gene | NCBI Accession No. | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| hk1 | NM_213252.1 | ACTTTGGGTGCAATCCTGAC | AGACGACGCACTGTTTTGTG |
| gck | NM_001045385.2 | TGAGGATGAAGAGCGAGGC | AGAGAAGGTGAATCCCAGCG |
| pklr | NM_201289.1 | CAAAGGACACTTCCCTGTAGAG | GGACAACGAGGACGATAACG |
| pck1 | NM_214751.1 | GTGAACTGAACCGAGACCTG | AGCACTTGAGAGCAAACGAT |
| fbp1a | NM_199942.2 | CATCTGTATGGGATTGCTGG | TTACCCCGTCTATCTGGCTC |
| g6p1a.1 | NM_001003512.2 | GCTGCACCATACGAGATGGA | TCACCAAACAGCACCCACTT |
| ppara | NM_0 01161333.1 | TGCTGGACTACCAGAACTGTGACA | TGCTGGCTGAGAACACTTCTGAG |
| pgc1a | XM_017357138.1 | AGTCTCCAAATGACCACAAGG | GGTTCTCTTGACTGGCTTTGT |
| pparg | NM_131467.1 | TTTTCCGCAGGACGATT | GAGGGAAGTATTTGAGATAGGAC |
| fasn | XM_021472581.1 | GGAGAATCTGACCCCACA | CTCCAAAACGACACCCAC |
| cyp2r1 | NM_001386362.1 | TGGAGAACTGATCATCGCGG | CCTCCACATACGGCATCCTC |
| cyp27b1 | NM_001311791.1 | AAGGCCGTCGTCAAGGAAAT | CTCGAGACGTGGCGTAATGA |
| cyp24a1 | NM_001089458.1 | CCTCCACATACGGCATCCTC | CCAAACGGCACATGAGCAAA |
| vdra | NM_130919.1 | GGATTCCACTTCAACGCC | CTCAGCCGAGGTTTACGA |
| vdrb | NM_001159985.1 | CAGTATGAAGCGGAAGGC | GGAGGTCTGAAGCGTGAA |
| insra | NM_001142672.1 | GGTGGGTGACAGGGTTCTTT | GCACACAGTCCGGATAACCT |
| insrb | NM_001123229.1 | TCATTTCACCCCTGCTGTGT | AGCAGCCGAAGTCTACATGG |
| gcgra | XM_021474732.1 | ATGAGCAGAGAAGCACCGAT | CAGGATGAAGGAGGCAAACA |
| gcgrb | XM_009295263.3 | CCACTACCAGAGCACACGAT | ACTCTTTGGGCACAGACTCA |
| cd36 | NM_001002363.1 | GCCTGTTGATGCTCTGGCTTCTC | CATTCCGACCACCCCCTGC |
| slc27a2a | NM_001025299.1 | CGTGCTTCTCCACACTCGAT | TGCATCCCGGTAAGTGTAGC |
| gip | NM_001080059.1 | TGCGCTGGTTTTGATTTGCC | TATCGGCGACTGAGCTTCTG |
| gipr | XM_005157739.4 | TGAGTGGGAAGACGGTGAA | CGGCTCGCAGGATGAATG |
| pyya | NM_001164371.1 | CGTCGCCACTGTCCTCA | TCCATACCGTTGCCTCGT |
| pyyb | NM_001327895.1 | CCACCCAAACCTGAACCT | CAAGTCTTCAACACGAGGC |
| sglt | NM_200681.1 | TAAAGCTGTCTGTGGAGCCGAAGT | ACAACATCAACCCTCGGAGACCAT |
| gcga | NM_001008595.3 | TGCCAGTCTTCTTTTGCTCC | CAGGTATTTGCTGTAGTCGTTG |
| gcgb | NM_001242770.1 | GGAGACCAGGAGAGCACAAG | TGCAGGTACGAGCTGACATC |
| pcsk1 | NM_001137662.1 | TTGGGCCGAACAGCAGTATGAGAAA | TGGATAAATGTCGGTGTGGTTCCAC |
| pcsk2 | NM_001142266.1 | CGCAAGAGAAACCCTGAAGC | TCTTGGAGGTCAGAACCGTC |
| actb2 | NM_181601.5 | GATGATGAAATTGCCGCACTG | ACCAACCATGACACCCTGATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Q.; Shao, R.; Wang, W.; Zhang, H.; Liao, X.; Wang, Z.; Yin, Z.; Ai, Q.; Mai, K.; Tang, X.; et al. Vitamin D3 Regulates Energy Homeostasis under Short-Term Fasting Condition in Zebrafish (Danio Rerio). Nutrients 2024, 16, 1271. https://doi.org/10.3390/nu16091271
Du Q, Shao R, Wang W, Zhang H, Liao X, Wang Z, Yin Z, Ai Q, Mai K, Tang X, et al. Vitamin D3 Regulates Energy Homeostasis under Short-Term Fasting Condition in Zebrafish (Danio Rerio). Nutrients. 2024; 16(9):1271. https://doi.org/10.3390/nu16091271
Chicago/Turabian StyleDu, Qingyang, Rui Shao, Wentao Wang, Hui Zhang, Xinmeng Liao, Zhihao Wang, Zhan Yin, Qinghui Ai, Kangsen Mai, Xiao Tang, and et al. 2024. "Vitamin D3 Regulates Energy Homeostasis under Short-Term Fasting Condition in Zebrafish (Danio Rerio)" Nutrients 16, no. 9: 1271. https://doi.org/10.3390/nu16091271