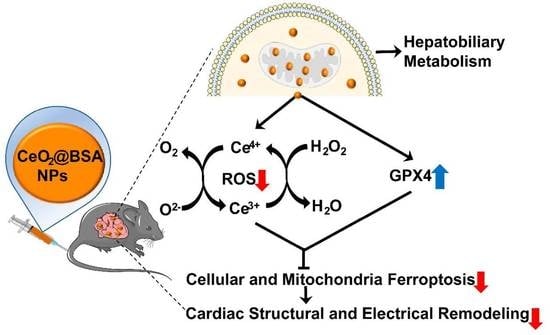
Biomimetic Nanozymes Suppressed Ferroptosis to Ameliorate Doxorubicin-Induced Cardiotoxicity via Synergetic Effect of Antioxidant Stress and GPX4 Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Synthesis of CeO2@BSA NPs
2.3. Nanoparticles Characterization
2.4. SOD Activity Assay and Catalase Activity Assay
2.5. Cellular Uptake Experiments
2.6. Cytotoxicity Assay
2.7. Experimental Design and Model Establishment
2.8. Determination of MDA, GSH, NT-proBNP, and cTnI in Mice Serum
2.9. Echocardiography
2.10. Surface ECG and Epicardial Electrical Mapping
2.11. Blood Pressure
2.12. Transmission Electron Microscope
2.13. Histology
2.14. Cell Culture and Cell Experiments
2.15. Cellular Mitochondrial Membrane Potential and ROS Measurement of H9c2 Cell Line
2.16. Mitochondrial Ferrous Ions and Mitochondrial Lipid Peroxidation Toxicity Assay
2.17. Western Blotting
2.18. Biodistribution and Biocompatibility Assessment
2.19. Statistical Analysis
3. Results and Discussion
3.1. CeO2@BSA NP Characterization
3.2. In Vitro Hybrid Enzymatic Mimetic Activity of CeO2@BSA NPs
3.3. CeO2@BSA NPs Protected DIC by Preventing Ferroptosis in Cardiomyocytes
3.4. Preventive and Therapeutic Efficacy of CeO2@BSA NPs on Doxorubicin-Induced Cardiac Structural and Electrical Remodeling in Mice
3.5. CeO2@BSA NPs Exhibited Protective Effect against Ferroptosis by Restoring GPX4 Expression and Improving Mitochondrial Function and Homeostasis
3.6. In Vivo Metabolism and Biocompatibility of CeO2@BSA NPs
3.7. Study Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armenian, S.; Xu, L.; Ky, B.; Sun, C.; Farol, L.; Pal, S.; Douglas, P.; Bhatia, S.; Chao, C. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016, 34, 1122–1130. [Google Scholar] [CrossRef]
- Lipshultz, S.; Adams, M.; Colan, S.; Constine, L.; Herman, E.; Hsu, D.; Hudson, M.; Kremer, L.; Landy, D.; Miller, T.; et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef] [Green Version]
- Rugbjerg, K.; Mellemkjaer, L.; Boice, J.; Køber, L.; Ewertz, M.; Olsen, J. Cardiovascular disease in survivors of adolescent and young adult cancer: A Danish cohort study, 1943–2009. J. Natl. Cancer Inst. 2014, 106, dju110. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.; Whaley, F.; Ewer, M. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Nabhan, C.; Byrtek, M.; Rai, A.; Dawson, K.; Zhou, X.; Link, B.; Friedberg, J.; Zelenetz, A.; Maurer, M.; Cerhan, J.; et al. Disease characteristics, treatment patterns, prognosis, outcomes and lymphoma-related mortality in elderly follicular lymphoma in the United States. Br. J. Haematol. 2015, 170, 85–95. [Google Scholar] [CrossRef]
- Young, R.; Ozols, R.; Myers, C. The anthracycline anti-neoplastic drugs. N. Engl. J. Med. 1981, 305, 139–153. [Google Scholar] [CrossRef]
- Dimarco, A.; Gaetani, M.; Orezzi, P.; Scarpinato, B.; Silvestrini, R.; Soldati, M.; Dasdia, T.; Valentini, L. ‘Daunomycin’, a new antibiotic of the rhodomycin group. Nature 1964, 201, 706–707. [Google Scholar] [CrossRef]
- Sawicki, K.; Sala, V.; Prever, L.; Hirsch, E.; Ardehali, H.; Ghigo, A. Preventing and Treating Anthracycline Cardiotoxicity: New Insights. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 309–332. [Google Scholar] [CrossRef]
- Ferrans, V.J. Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer Treat. Rep. 1978, 62, 955–961. [Google Scholar]
- Berry, G.; Jorden, M. Pathology of radiation and anthracycline cardiotoxicity. Pediatr. Blood Cancer 2005, 44, 630–637. [Google Scholar] [CrossRef]
- Jones, L.; Haykowsky, M.; Peddle, C.; Joy, A.; Pituskin, E.; Tkachuk, L.; Courneya, K.; Slamon, D.; Mackey, J. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1026–1031. [Google Scholar] [CrossRef] [Green Version]
- Amioka, M.; Sairaku, A.; Ochi, T.; Okada, T.; Asaoku, H.; Kyo, T.; Kihara, Y. Prognostic Significance of New-Onset Atrial Fibrillation in Patients with Non-Hodgkin’s Lymphoma Treated with Anthracyclines. Am. J. Cardiol. 2016, 118, 1386–1389. [Google Scholar] [CrossRef]
- Mazur, M.; Wang, F.; Hodge, D.; Siontis, B.; Beinborn, D.; Villarraga, H.; Lerman, A.; Friedman, P.; Herrmann, J. Burden of Cardiac Arrhythmias in Patients with Anthracycline-Related Cardiomyopathy. JACC Clin. Electrophysiol. 2017, 3, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kilickap, S.; Barista, I.; Akgul, E.; Aytemir, K.; Aksoy, S.; Tekuzman, G. Early and late arrhythmogenic effects of doxorubicin. South. Med. J. 2007, 100, 262–265. [Google Scholar] [CrossRef]
- Fradley, M.; Viganego, F.; Kip, K.; Martin, A.; Patel, A.; Ismail-Khan, R.; Chae, S.; Herweg, B.; Labovitz, A. Rates and risk of arrhythmias in cancer survivors with chemotherapy-induced cardiomyopathy compared with patients with other cardiomyopathies. Open Heart 2017, 4, e000701. [Google Scholar] [CrossRef] [Green Version]
- Cvetkokic, R.; Scott, L. Dexrazoxane—A review of its use for cardioprotection during anthracycline chemotherapy. Drugs 2005, 65, 1005–1024. [Google Scholar] [CrossRef]
- Seifert, C.; Nesser, M.; Thompson, D. Dexrazoxane in the prevention of doxorubicin-induced cardiotoxicity. Ann. Pharmacother. 1994, 28, 1063–1072. [Google Scholar] [CrossRef]
- Octavia, Y.; Tocchetti, C.; Gabrielson, K.; Janssens, S.; Crijns, H.; Moens, A. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.; Lyu, Y.; Liu, L.; Yeh, E. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Cui, M.; Jin, L.; Wang, Y.; Lv, F.; Liu, Y.; Zheng, W.; Shang, H.; Zhang, J.; et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 2016, 22, 175–182. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.N.; Mutharasan, R.; Naik, T.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Lebrecht, D.; Setzer, B.; Ketelsen, U.; Haberstroh, J.; Walker, U. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation 2003, 108, 2423–2429. [Google Scholar] [CrossRef] [Green Version]
- Kitakata, H.; Endo, J.; Ikura, H.; Moriyama, H.; Shirakawa, K.; Katsumata, Y.; Sano, M. Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis. Int. J. Mol. Sci. 2022, 23, 1414. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, C.; Su, X.; Zhang, J.; Gokulnath, P.; Vulugundam, G.; Li, G.; Yang, X.; An, N.; Liu, C.; et al. Relevance of Ferroptosis to Cardiotoxicity Caused by Anthracyclines: Mechanisms to Target Treatments. Front. Cardiovasc. Med. 2022, 9, 896792. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020, 5, e132747. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, J.; Li, J.; Huang, L.; Yang, J.; Huang, K.; Li, H.; Jiang, N.; Zheng, S.; Zhang, X.; et al. Tumor-Targeted and Clearable Human Protein-Based MRI Nanoprobes. Nano Lett. 2017, 17, 4096–4100. [Google Scholar] [CrossRef]
- Neal, C.; Das, S.; Saraf, S.; Tetard, L.; Seal, S. Self-Assembly of PEG-Coated Ceria Nanoparticles Shows Dependence on PEG Molecular Weight and Ageing. Chempluschem 2015, 80, 1680–1690. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yan, X.; Fan, J.; Li, D.; Ren, J.; Qu, X. A MXene-derived redox homeostasis regulator perturbs the Nrf2 antioxidant program for reinforced sonodynamic therapy. Chem. Sci. 2022, 13, 6704–6714. [Google Scholar] [CrossRef]
- Yu, Z.; Lou, R.; Pan, W.; Li, N.; Tang, B. Nanoenzymes in disease diagnosis and therapy. Chem. Commun. 2020, 56, 15513–15524. [Google Scholar] [CrossRef]
- Shioji, K.; Oyama, Y.; Okuma, K.; Nakagawa, H. Synthesis and properties of fluorescence probe for detection of peroxides in mitochondria. Bioorg. Med. Chem. Lett. 2010, 20, 3911–3915. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Celutkiene, J.; Henriksen, P.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Song, M.; Chen, F.; Li, Y.; Zhang, L.; Wang, F.; Qin, R.; Wang, Z.; Zhong, M.; Tang, M.; Zhang, W.; et al. Trimetazidine restores the positive adaptation to exercise training by mitigating statin-induced skeletal muscle injury. J. Cachexia Sarcopenia Muscle 2018, 9, 106–118. [Google Scholar] [CrossRef]
- Sun, M.; Sang, Y.; Deng, Q.; Liu, Z.; Ren, J.; Qu, X. Specific generation of nitric oxide in mitochondria of cancer cell for selective oncotherapy. Nano Res. 2022, 15, 5273–5278. [Google Scholar] [CrossRef]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 10, 1056–1058. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.; King, J.; Seal, S.; Self, W. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Wang, K.; Kolattukudy, P. Cerium oxide nanoparticles inhibit oxidative stress and nuclear factor-kappaB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharm. Exp. 2011, 338, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.; Peden, C. Chemistry. Oxygen vacancies and catalysis on ceria surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef]
- Celardo, I.; Traversa, E.; Ghibelli, L. Cerium oxide nanoparticles: A promise for applications in therapy. J. Exp. Ther. Oncol. 2011, 9, 47–51. [Google Scholar]
- Sangomla, S.; Saifi, M.; Khurana, A.; Godugu, C. Nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. J. Trace Elem. Med. Biol. 2018, 47, 53–62. [Google Scholar] [CrossRef]
- Cai, R.; Xiao, L.; Qiu, J.; Zhao, L.; Li, Z.; Ju, H.; Sun, M.; Zhu, W.; Wang, Z.; Du, F. Fabrication of cerium doped carbon dots with highly radical scavenging activity alleviates ferroptosis-induced oxidative damage. Nanotechnology 2021, 32, 395605. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, H.; Mercer, R.; Barger, M.; Rao, M.; Meighan, T.; Schwegler-Berry, D.; Castranova, V.; Ma, J. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats. Nanotoxicology 2011, 5, 312–325. [Google Scholar] [CrossRef]
- Ma, J.; Mercer, R.; Barger, M.; Schwegler-Berry, D.; Scabilloni, J.; Ma, J.; Castranova, V. Induction of pulmonary fibrosis by cerium oxide nanoparticles. Toxicol. Appl. Pharm. 2012, 262, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, M.; Sandhage, K.; Naik, R. Protein- and Peptide-Directed Syntheses of Inorganic Materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef]
- Weiner, S.; Addadi, L. Design strategies in mineralized biological materials. J. Mater. Chem. 1997, 7, 689–702. [Google Scholar] [CrossRef]
- Xie, S.; Yin, G.; Pu, X.; Hu, Y.; Huang, Z.; Liao, X.; Yao, Y.; Chen, X. Biomimetic Mineralization of Tumor Targeted Ferromagnetic Iron Oxide Nanopartieles Used for Media of Magnetic Hyperthermia. Curr. Drug Deliv. 2017, 14, 349–356. [Google Scholar] [CrossRef]
- Yang, W.; Guo, W.; Chang, J.; Zhang, B. Protein/peptide-templated biomimetic synthesis of inorganic nanoparticles for biomedical applications. J. Mater. Chem. B 2017, 5, 401–417. [Google Scholar] [CrossRef]
- Xiao, B.; Zhou, X.; Xu, H.; Wu, B.; Hu, D.; Hu, H.; Pu, K.; Zhou, Z.; Liu, X.; Tang, J.; et al. Integration of Polymerization and Biomineralization as a Strategy to Facilely Synthesize Nanotheranostic Agents. ACS Nano 2018, 12, 12682–12691. [Google Scholar] [CrossRef]
- Gradishar, W.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Chung, S.; Nafiujjaman, M.; Hill, M.; Siziba, M.; Contag, C.; Kim, T. Ceria-based nanotheranostic agent for rheumatoid arthritis. Theranostics 2020, 10, 11863–11880. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Ke, H.; Zhu, A.; Wang, Y.; Wang, J.; Shen, J.; Liu, G.; Chen, C.; Zhao, Y.; et al. Smart Albumin-Biomineralized Nanocomposites for Multimodal Imaging and Photothermal Tumor Ablation. Adv. Mater. 2015, 27, 3874–3882. [Google Scholar] [CrossRef]
- Carvalho, F.; Burgeiro, A.; Garcia, R.; Moreno, A.; Carvalho, R.; Oliveira, P. Doxorubicin-induced cardiotoxicity: From bioenergetic failure and cell death to cardiomyopathy. Med. Res. Rev. 2014, 34, 106–135. [Google Scholar] [CrossRef]
- Huo, X.; Wang, C.; Yu, Z.; Peng, Y.; Wang, S.; Feng, S.; Zhang, S.; Tian, X.; Sun, C.; Liu, K.; et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 2017, 62, e12390. [Google Scholar] [CrossRef]
- Horacek, J.; Jakl, M.; Horackova, J.; Pudil, R.; Jebavy, L.; Maly, J. Assessment of anthracycline-induced cardiotoxicity with electrocardiography. Exp. Oncol. 2009, 31, 115–117. [Google Scholar] [PubMed]
- Liang, H.; Wu, X.; Zhao, G.; Feng, K.; Ni, K.; Sun, X. Renal Clearable Ultrasmall Single-Crystal Fe Nanoparticles for Highly Selective and Effective Ferroptosis Therapy and Immunotherapy. J. Am. Chem. Soc. 2021, 143, 15812–15823. [Google Scholar] [CrossRef]
- Prathumsap, N.; Ongnok, B.; Khuanjing, T.; Arinno, A.; Maneechote, C.; Apaijai, N.; Chunchai, T.; Arunsak, B.; Kerdphoo, S.; Janjek, S.; et al. Vagus nerve stimulation exerts cardioprotection against doxorubicin-induced cardiotoxicity through inhibition of programmed cell death pathways. Cell. Mol. Life Sci. CMLS 2022, 80, 21. [Google Scholar] [CrossRef]
- Li, N.; Jiang, W.; Wang, W.; Xiong, R.; Wu, X.; Geng, Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol. Res. 2021, 166, 105466. [Google Scholar] [CrossRef]
- Oh, S.; Ikeda, M.; Ide, T.; Hur, K.; Lee, M. Mitochondrial event as an ultimate step in ferroptosis. Cell Death Discov. 2022, 8, 414. [Google Scholar] [CrossRef]
- Lewerenz, J.; Ates, G.; Methner, A.; Conrad, M.; Maher, P. Oxytosis/Ferroptosis-(Re-) Emerging Roles for Oxidative Stress-Dependent Non-apoptotic Cell Death in Diseases of the Central Nervous System. Front. Neurosci. 2018, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Fefelova, N.; Pamarthi, S.; Gwathmey, J. Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease. Cells 2022, 11, 2726. [Google Scholar] [CrossRef]
- Lopez-Crisosto, C.; Pennanen, C.; Vasquez-Trincado, C.; Morales, P.; Bravo-Sagua, R.; Quest, A.; Chiong, M.; Lavandero, S. Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology. Nat. Rev. Cardiol. 2017, 14, 342–360. [Google Scholar] [CrossRef]
- Serasinghe, M.; Chipuk, J. Mitochondrial Fission in Human Diseases. Handb. Exp. Pharm. 2017, 240, 159–188. [Google Scholar] [CrossRef]
- Ekstrand, M.; Falkenberg, M.; Rantanen, A.; Park, C.; Gaspari, M.; Hultenby, K.; Rustin, P.; Gustafsson, C.; Larsson, N. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004, 13, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Herzig, S.; Shaw, R. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Zablocki, D.; Sadoshima, J. The role of Drp1 in mitophagy and cell death in the heart. J. Mol. Cell. Cardiol. 2020, 142, 138–145. [Google Scholar] [CrossRef]
- Wang, T.; Yuan, C.; Liu, J.; Deng, L.; Li, W.; He, J.; Liu, H.; Qu, L.; Wu, J.; Zou, W. Targeting Energy Protection as a Novel Strategy to Disclose Di’ao Xinxuekang against the Cardiotoxicity Caused by Doxorubicin. Int. J. Mol. Sci. 2023, 24, 897. [Google Scholar] [CrossRef]
- He, H.; Wang, L.; Qiao, Y.; Yang, B.; Yin, D.; He, M. Epigallocatechin-3-gallate pretreatment alleviates doxorubicin-induced ferroptosis and cardiotoxicity by upregulating AMPKα2 and activating adaptive autophagy. Redox Biol. 2021, 48, 102185. [Google Scholar] [CrossRef]
- Casals, E.; Zeng, M.; Parra-Robert, M.; Fernandez-Varo, G.; Morales-Ruiz, M.; Jimenez, W.; Puntes, V.; Casals, G. Cerium Oxide Nanoparticles: Advances in Biodistribution, Toxicity, and Preclinical Exploration. Small 2020, 16, e1907322. [Google Scholar] [CrossRef]
- Kolli, M.; Manne, N.; Para, R.; Nalabotu, S.; Nandyala, G.; Shokuhfar, T.; He, K.; Hamlekhan, A.; Ma, J.; Wehner, P.; et al. Cerium oxide nanoparticles attenuate monocrotaline induced right ventricular hypertrophy following pulmonary arterial hypertension. Biomaterials 2014, 35, 9951–9962. [Google Scholar] [CrossRef] [Green Version]
- Kuruvilla, L.; Kartha, C. Cerium depresses endocardial endothelial cell-mediated proliferation of cardiac fibroblasts. Biol. Trace Elem. Res. 2006, 114, 85–92. [Google Scholar] [CrossRef]
- Nair, R.; Preeta, R.; Smitha, G.; Adiga, I. Variation in mitogenic response of cardiac and pulmonary fibroblasts to cerium. Biol. Trace Elem. Res. 2003, 94, 237–246. [Google Scholar] [CrossRef]
- Jankauskas, S.; Kansakar, U.; Sardu, C.; Varzideh, F.; Avvisato, R.; Wang, X.; Matarese, A.; Marfella, R.; Ziosi, M.; Gambardella, J.; et al. COVID-19 Causes Ferroptosis and Oxidative Stress in Human Endothelial Cells. Antioxidants 2023, 12, 326. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, S.; Peng, J.; Cheng, S.; Zhang, Q.; Zhang, N.; Zhou, Z.; Zhang, Y.; Zhao, Y.; Liu, T. Biomimetic Nanozymes Suppressed Ferroptosis to Ameliorate Doxorubicin-Induced Cardiotoxicity via Synergetic Effect of Antioxidant Stress and GPX4 Restoration. Nutrients 2023, 15, 1090. https://doi.org/10.3390/nu15051090
Zhang Y, Liu S, Peng J, Cheng S, Zhang Q, Zhang N, Zhou Z, Zhang Y, Zhao Y, Liu T. Biomimetic Nanozymes Suppressed Ferroptosis to Ameliorate Doxorubicin-Induced Cardiotoxicity via Synergetic Effect of Antioxidant Stress and GPX4 Restoration. Nutrients. 2023; 15(5):1090. https://doi.org/10.3390/nu15051090
Chicago/Turabian StyleZhang, Yunpeng, Shuang Liu, Jing Peng, Shifeng Cheng, Qingling Zhang, Nan Zhang, Zandong Zhou, Yue Zhang, Yang Zhao, and Tong Liu. 2023. "Biomimetic Nanozymes Suppressed Ferroptosis to Ameliorate Doxorubicin-Induced Cardiotoxicity via Synergetic Effect of Antioxidant Stress and GPX4 Restoration" Nutrients 15, no. 5: 1090. https://doi.org/10.3390/nu15051090