Vegetable as a Source of Bioactive Compounds with Photoprotective Properties: Implication in the Aging Process
Abstract
:1. Introduction
2. The Skin as the First Protective Barrier against UV Radiation
2.1. Generation of Reactive Oxygen Species in Skin Aging
2.2. Skin Cancers
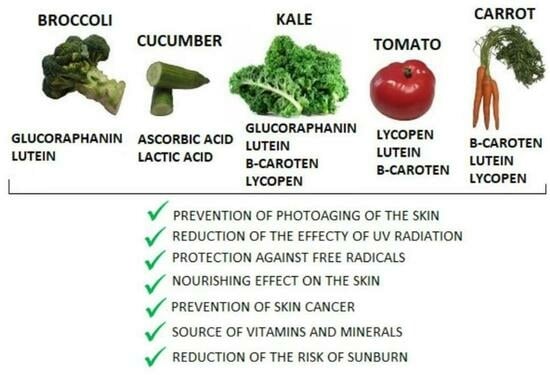
3. Vegetables as a Source of Photoprotective Ingredients
3.1. Broccoli
3.2. Cucumber
3.3. Kale
3.4. Tomato
3.5. Carrot
4. Photoprotective Bioactive Food Ingredients
4.1. Glucoraphanin
4.2. Lycopene
4.3. Luteolin
4.4. β-Carotene
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kilian-Pięta, E.; Hopp, M. Wpływ melanogenezy na powstawanie przebarwień. Kosmetol. Estet. 2019, 8, 419–420. [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Ata, P.; Majewski, S. Fotostarzenie skóry. Przegląd Dermatol. 2013, 100, 178–183. [Google Scholar]
- Ebisz, M.; Brokowska, M. Szkodliwe oddziaływanie promieniowania ultrafioletowego na skórę człowieka. Hygeia Public Health 2015, 50, 467–473. [Google Scholar]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S. Voorhees Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet radiation exposure and its impact on skin cancer risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Maddodi, N.; Jayanthy, A.; Setalur, V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef]
- Wolski, T.; Kędzia, B. Budowa i fizjologia skóry. In Farmakoterapia skóry. Cz. 1; Publisher Post Fitoter: Lublin, Poland, 2019; Volume 20, Issue 1; pp. 61–67. [Google Scholar]
- Geoffrey, K.; Mwangi, A.N.; Maru, S.M. Sunscreen products: Rationale for use, formulation development and regulatory considerations. Saudi Pharm. J. 2019, 27, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The efficacy and safety of sunscreen use for the prevention of skin cancer. CMAJ 2020, 192, E1802–E1808. [Google Scholar] [CrossRef]
- Tsai, J.; Chien, A.L. Photoprotection for skin of color. Am. J. Clin. Dermatol. 2022, 23, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Charakterystyka oleju z nasion malin (Rubus idaeus L.). Chem. Spożywcza 2000, 69, 187–193. [Google Scholar]
- Sarruf, F.D.; Sauce, R.; Candido, T.M.; Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Butyrospermum parkii butter increased the photostability and in vivo SPF of a molded sunscreen system. J. Cosmet. Dermatol. 2020, 19, 3296–3301. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Jose, J. Exploring the photo protective potential of solid lipid nanoparticle-based sunscreen cream containing Aloe vera. Environ. Sci. Pollut. Res. 2020, 27, 20876–20888. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.F. Topical and systemic photoprotection. Dermatol. Ther. 2003, 16, 8–15. [Google Scholar] [CrossRef]
- Evans, J.A.; Johnson, E.J. The role of phytonutrients in skin health. Nutrients 2010, 2, 903–928. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Fuchs, E. Building and maintaining the skin. Cold Spring Harb. Perspect. Biol. 2022, 14, a040840. [Google Scholar] [CrossRef] [PubMed]
- Greinert, R.; de Vries, E.; Erdmann, F.; Espina, C.; Auvinen, A.; Kesminiene, A.; Schüz, J. European code against cancer 4th edition: Ultraviolet radiation and cancer. Cancer Epidemiol. 2015, 39, 75–83. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced infammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Dixon, K.M.; Tongkao-On, W.; Sequeira, V.B.; Carter, S.E.; Song, E.J.; Rybchyn, M.S.; Gordon-Thomson, C.; Mason, R.S. Vitamin D and death by sunshine. Int. J. Mol. Sci. 2013, 14, 1964–1977. [Google Scholar] [CrossRef] [PubMed]
- Estimated Number of New Skin Cancer Cases in the U.S. in 2023, by Gender. Available online: https://www.statista.com/statistics/663036/number-of-new-skin-cancer-cases-in-the-us-by-gender/ (accessed on 4 July 2023).
- Per Capita Consumption of Fresh Broccoli in the United States from 2000 to 2022. Available online: https://www.statista.com/statistics/257338/per-capita-consumption-of-fresh-broccoli-in-the-us/ (accessed on 4 July 2023).
- Jeffery, E.H.; Brown, A.F.; Kurililch, A.C.; Keck, A.S.; Matusheski, N.; Klein, B.P.; Juvik, J.A. Variation in content of bioactive components in broccoli. J. Food Compos. Anal. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- Finley, J.W.; Ip, C.; Lisk, D.J.; Davis, C.D.; Hintze, K.J.; Whanger, P.D. Cancer-protective properties of high-selenium broccoli. J. Agric. Food Chem. 2001, 49, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M. Traditional phytotherapy in central Italy (Marche, Abruzzo, and latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Jenkins, S.N.; Fahey, J.W.; Ye, L.; Wehagc, S.L.; Liby, K.T.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006, 240, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Benedict, A.L.; Jenkins, S.N.; Ye, L.; Wehage, S.L.; Talalay, P. Dietary glucoraphanin-rich broccoli sprout extracts protect against UV radiation-induced skin carcinogenesis in SKH-1 hairless mice. Photochem. Photobiol. Sci. 2010, 9, 597–600. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Wade, K.L.; Jenkins, S.N.; Shapiro, T.A.; Fuchs, E.J.; Kerns, M.L.; Talalay, P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol. Biomark. Prev. 2007, 16, 847–851. [Google Scholar] [CrossRef]
- Lim, S.H.; Jung, S.K.; Byun, S.; Lee, E.J.; Hwang, J.A.; Seo, S.G.; Kim, Y.A.; Yu, J.G.; Lee, K.W.; Lee, H.J. Luteolin suppresses UVB-induced photoageing by targeting JNK1 and p90 RSK2. J. Cell. Mol. Med. 2013, 17, 672–680. [Google Scholar] [CrossRef]
- Kerns, M.L.; Chien, A.L.; Kang, S. A Role for NRF2-signaling in the treatment and prevention of solar lentigines. Plast. Reconstr. Surg. 2021, 148, 27–31. [Google Scholar] [CrossRef]
- Saw, C.L.; Huang, M.-T.; Liu, Y.; Khor, T.O.; Conney, A.H.; Kong, A.-N. Impact of Nrf2 on UVB-induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol. Carcinog. 2010, 50, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Fahey, J.W.; Healy, Z.R.; Wehage, S.L.; Benedict, A.L.; Min, C.; Dinkova-Kostova, A.T. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 17500–17505. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T. The isothiocyanate sulforaphane induces the phase 2 response by signaling of the Keap1-Nrf2-are pathway: Implications for dietary protection against cancer. In Dietary Modulation of Cell Signaling Pathways, 1st ed.; Surh, Y.-J., Dong, Z., Cadenas, E., Packer, L., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 205–227. [Google Scholar]
- Alyoussef, A.; Taha, M. Antitumor activity of sulforaphane in mice model of skin cancer via blocking sulfatase-2. Exp. Dermatol. Cancer Lett. 2018, 240, 243–252. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef]
- Moreno, D.A.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef]
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef] [PubMed]
- Ozyurek, M.; Bektasoglu, B.; Guclu, K.; Apak, R. Measurement of xanthine oxidase inhibition activity of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method. Anal. Chim. Acta 2009, 636, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean diet: Citrus, cucumber and grape. J. Prev. Med. Hyg. 2022, 63, 21–27. [Google Scholar]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and therapeutic potential of cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Sotiroudis, G.; Melliou, E.; Sotiroudis, T.G.; Chinou, I. Chemical analysis, antioxidant and antimicrobial activity of three Greek cucumber (Cucumis sativus) cultivars. J. Food Biochem. 2010, 34, 61–78. [Google Scholar] [CrossRef]
- Gill, N.S.; Garg, M.; Bansal, R.; Sood, S.; Muthuraman, A.; Bali, M.; Sharma, P.D. Evaluation of antioxidant and antiulcer potential of Cucumis sativum L. seed extract in rats. Asian J. Clin. Nutr. 2009, 1, 131–138. [Google Scholar] [CrossRef]
- Kai, H.; Baba, M.; Okuyama, T. Inhibitory effect of Cucumis sativus on melanin production in melanoma B16 cells by downregulation of tyrosinase expression. Planta Med. 2008, 74, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Glew, R.H.; Millson, M.; Huang, H.S.; Chuang, L.T.; Sanz, C.; Hayırlıoglu-Ayaz, S. Nutrient contents of kale (Brassica oleraceae L. var. acephala DC.). Food Chem. 2006, 96, 572–579. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef]
- Cooperstone, J.L.; Tober, K.L.; Riedl, K.M.; Teegarden, M.D.; Cichon, M.J.; Francis, D.M.; Schwartz, S.J.; Oberyszyn, T.M. Tomatoes protect against development of UV-induced keratinocyte carcinoma via metabolomic alterations. Sci. Rep. 2017, 7, 5106. [Google Scholar] [CrossRef]
- Fazekas, Z.; Gao, D.; Saladi, R.N.; Lu, Y.; Lebwohl, M.; Wei, H. Protective effects of lycopene against ultraviolet b-induced photodamage. Nutr. Cancer 2003, 47, 181–187. [Google Scholar] [CrossRef]
- Heo, H.; Madhavan, J.; Eun, S.; Jung, H.; Lee, H. Pre-Clinical evaluation of proprietary lutein, zeaxanthin, and rosemary formulation for its dermal protective activity in male swiss albino mice. Prev. Nutr. Food Sci. 2021, 26, 425–433. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Chawalitpong, S.; Ichikawa, S.; Uchibori, Y.; Nakamura, S.; Katayama, S. Long-Term Intake of Glucoraphanin-Enriched Kale Suppresses Skin Aging via Activating Nrf2 and the TβRII/Smad Pathway in SAMP1 Mice. J. Agric. Food Chem. 2019, 67, 9782–9788. [Google Scholar] [CrossRef]
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E. Influences of orally taken carotenoid-rich curly kale extract on collagen I/elastin index of the skin. Nutrients 2017, 9, 775. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A Review. Foods 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Kurina, A.B.; Solovieva, A.E.; Khrapalova, I.A.; Artemyeva, A.M. Biochemical composition of tomato fruits of various colors. Vavilov J. Genet. Breed. 2021, 25, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Heinrich, U.; Wiseman, S.; Eichler, O.; Sies, H.; Tronnier, H. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J. Nutr. 2001, 131, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Khomich, L.M.; Perova, I.B.; Eller, K.I. Carrot juice nutritional profile. Vopr. Pitan. 2020, 89, 86–95. [Google Scholar]
- Gröber, U. Mikroskładniki Odżywcze. Tuning Metaboliczny—Profilaktyka—Leczenie; MedPharm Polska: Wrocław, Poland, 2010. [Google Scholar]
- Stahl, W.; Heinrich, U.; Aust, O.; Tronnier, H.; Sies, H. Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 2006, 5, 238–242. [Google Scholar] [CrossRef]
- Singh, S.; Lohani, A.; Mishra, A.K.; Verma, A. Formulation and evaluation of carrot seed oil-based cosmetic emulsions. J. Cosmet. Laser Ther. 2019, 21, 99–107. [Google Scholar] [CrossRef]
- Bianconi, M.; Ceriotti, L.; Cuzzocrea, S.; Esposito, E.; Pressi, G.; Sgaravatti, E.; Bertaiola, O.; Guarnerio, C.; Barbieri, E.; Semenzato, A.; et al. Red carrot cells cultured in vitro are effective, stable, and safe ingredients for skin care, nutraceutical, and food applications. Front. Bioeng. Biotechnol. 2020, 8, 575079. [Google Scholar] [CrossRef]
- Benedict, A.L.; Knatko, E.V.; Dinkova-Kostova, A.T. The indirect antioxidant sulforaphane protects against thiopurine-mediated photooxidative stress. Carcinogenesis 2012, 33, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Eicker, J.; Kürten, V.; Wild, S.; Riss, G.; Goralczyk, R.; Krutmann, J.; Berneburg, M. Betacarotene supplementation protects from photoaging-associated mitochondrial DNA mutation. Photochem. Photobiol. Sci. 2003, 2, 655–659. [Google Scholar] [CrossRef]
- Bando, N.; Hayashi, H.; Wakamatsu, S.; Inakuma, T.; Miyoshi, M.; Nagao, A.; Yamauchi, R.; Terao, J. Participation of singlet oxygen in ultraviolet-a-induced lipid peroxidation in mouse skin and its inhibition by dietary beta-carotene: An ex vivo study. Free Radic. Biol. Med. 2004, 37, 1854–1863. [Google Scholar] [CrossRef]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E.; Rhodes, L.E. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: A randomized controlled trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef]
- Aust, O.; Stahl, W.; Sies, H.; Tronnier, H.; Heinrich, U. supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against uv-light-induced erythema. Int. J. Vitam. Nutr. Res. 2005, 75, 54–60. [Google Scholar] [CrossRef]
- Cho, S.; Lee, D.H.; Won, C.-H.; Kim, S.M.; Lee, S.; Lee, M.-J.; Chung, J.H. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type i procollagen gene expression in human skin in vivo. Dermatology 2010, 221, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jiang, S.; Levine, N.; Watson, R.R. Carotenoid supplementation reduces erythema in human skin after simulated solar radiation exposure. Proc. Soc. Exp. Biol. Med. 2000, 223, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. sulforaphane bioavailability from glucoraphanin-rich broccoli: Control by active endogenous myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed]
- Ascenso, A.; Pedrosa, T.; Pinho, S.; Pinho, F.; Oliveira, J.M.; Cabral Marques, H.; Oliveira, H.; Simões, S.; Santos, C. The Effect of Lycopene Preexposure on UV-B-Irradiated Human Keratinocytes. Oxidative Med. Cell. Longev. 2016, 2016, 8214631. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.; Stanghellini, E.; Ettorre, A.; Di Stefano, A.; Andreassi, L. Antioxidant activity of topically applied lycopene. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 52–55. [Google Scholar] [CrossRef]
- Que, F.; Hou, X.L.; Wang, G.L.; Xu, Z.S.; Tan, G.F.; Li, T.; Wang, Y.H.; Khadr, A.; Xiong, A.S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and zeaxanthin and their roles in age-related macular degeneration-neurodegenerative disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Type of Extract | Type of Study | Activity | References |
|---|---|---|---|---|
| Sulphoraphane | Broccoli sprout extracts | In vivo/oral aplicated/ SKH-1 female hairless mice | ↓ incidence, number and volume of the tumor ↑ Protection against UV radiation | [28] |
| Broccoli sprout extracts (in 80% acetone) containing 1 mmol of 0.3 μmol sulforaphane or carrier | In vitro/ Human HaCaT cells In vivo/topical aplicated/ female SKH-1 hairless mice/mouse PE keratinocytes | ↓ number and volume of the tumor ↑ NQO1, glutathione ↑ inhibition of iNOS | [27] | |
| Broccoli sprout extracts (in 80% acetone:20% water by volume) | In vivo/topical aplicated/ SKH-1 female hairless mice | ↑ protein levels of NAD(P)H:quinone ↑ oxidoreductase 1 (NQO1) ↑ glutathione S-transferase A1 ↑ heme oxygenase 1 | [29] | |
| Broccoli sprout extract | In vivo/topical aplicated/ SKH-1 hairless mice | ↑ phase 2 enzymes ↑ protect against UVR-induced inflammation and edema in mice ↓ susceptibility to erythema (311-nm UVR) in humans | [33] | |
| Broccoli sprout extract | In vivo/topical aplicated/ WT and Nrf2–/–C57BL/C57BL/6 male mice | ↑ NRF2, ↓ mottled hyperpigmentation of the treated areas ↓ 50% melanin deposition ↓ 30% reduction in the fold change of tyrosinase expression | [31] | |
| Sulforaphane (synthetic) | In vitro/ dermal fibroblasts or keratinocytes isolated from SKH-1 hairless mice | ↑ NQO1 ↓ ROS caused by UV radiation | [62] | |
| Sulforaphane (synthetic) | In vivo/topical and oral aplicated/ Swiss albino mice | ↑ NRF2 ↓ sulfatase-1, 2 ↓ gene and protein expression TNF-α, IL-1β, caspase-3 blocked sulfatase-2 activity ↑ HSPGs, G7 ↓ glypican-3 | [35] | |
| Sulforaphane (synthetic) | In vivo/topical aplicated/ Nrf2 KO, WT C57BL/6 mice | ↑ NRF2 ↑ Il-1β, IL-6 | [32] | |
| Glucoraphanin | Spray-dried kale and GEK juice (Glucoraphanin) | In vivo/oral aplicated/ male SAMP1 mice | ↑ suppressed the thinning of the dorsal skin layer ↑ collagen production a ↑ Nrf2 and HO-1 expression level ↑ TβRII, Smad3 expressions | [53] |
| Lycopene | Powdered tomatoes (feed with 10% lycopene) | In vivo/oral aplicated/ male and female SKH-1 hairless mice | ↑ carotnoids in the skin and plasma ↓ number of emerging tumors ↓ inflammation and subsequent DNA damage in the skin | [49] |
| Lycopene in acetone | In vivo/topical aplicated/ SKH-1 hairless mice | ↑ inhibition of epidermal ornithine decarboxylase activity ↑ inhibition of andmyeloperoxidase | [50] | |
| Luteolin | Luteolin (98%) was purchased from Indofine Chemical | In vitro/ Human HaCaT cells In vivo/topical aplicated/ SKH-1 hairless mice | ↑ inhibition UVB-induced MMP-1 expression in HaCaT cells ↑ inhibition of JNK1 and p90RSK2 activity ↑ inhibition of AP-1 | [30] |
| Patented supplement (lutein, zeaxanthin and rosemary) dispersed in sunflower oil XanMax® 80 | In vivo/topical aplicated/ Swiss albino mice | ↑ protective effect skin dehydration due to UV radiation | [51] | |
| B-caroten | Stock solutions of betacarotene | In vitro/ Human fibroblast culture from a human foreskin | ↑ protection against mtDNA mutation, which proves the prevention of photoaging ↓ singlet oxygen quencher | [63] |
| β-Carotene, β-apo-8V-carotenal, and δ-tocopherol, | In vivo/oral aplicated/ male BALB mice | ↑ increase β-carotene in TBARS ↑ prevention UVA-induced lipid peroxidation | [64] |
| Compounds | Type of Extract | Group Characteristics and Type of Application | Effect | References |
|---|---|---|---|---|
| Sulphoraphane | Broccoli sprout extracts (in 80% acetone:20% water by volume) | Human subject/ topical aplicated—17 patients, aged 25–51 years | ↑ protein levels of NAD(P)H:quinone ↑ oxidoreductase 1 (NQO1) ↑ glutathione S-transferase A1 ↑heme oxygenase 1 | [29] |
| Broccoli sprout extract | Human subject/ topical applicated—6 patients, aged 28–53 years | ↑ phase 2 enzymes ↑ protect against UVR-induced inflammation and edema ↓ susceptibility to erythema (311-nm UVR) in humans | [33] | |
| Broccoli sprout extract | Human subject/ topical aplicated—7 patients | ↑NRF2, ↓ mottled hyperpigmentation of the treated areas ↓ 50% melanin deposition ↓ 30% reduction in the fold change of tyrosinase expression | [31] | |
| Likopen | Tomatoe paste 55 g with 10 g olive oil on white bread | Human subject/ oral aplicated—20 patients, aged 21–47 years | ↓ ROS ↓ MMP-1 ↓ mtDNA 3895-bp deletion ↑ procollagen deposition | [65] |
| Gel–emulsion containing lycopene, and gel-emulsjon containing Vit. C and E | Human subject/ topical aplicated—10 patients, aged 20–42 years | ↑ protective effect against UV-induced erythema | [65] | |
| Tomatoe puree + olive oil carrot juice lycopene supplement with tomatoe extract Lycopene drink (from tomatoe extract) and synthetic lycopene | Human subject/ oral aplicated—22 patients, aged 26–67 years | ↑ protection against UV-induced erythema | [56,59] | |
| 1. Capsules with synthetic lycopene. 2. Tomatoe extract capsule. 3. 250 mL solubilized lycopene drink | Human subject/ oral aplicated—36 patients | ↑ protective effect against UV-induced erythema | [66] | |
| B-carotene | Carotene filled in a soft capsule | Human subject/oral aplication—30 patients, over the age of 50 years | ↑ expression of type I proctocollagen gene and protein ↓ facial wrinkles ↓r UV-induced erythema and DNA damage | [67] |
| Supplementation with 30 mg of natural carotenoids containing 29.4 mg of beta-carotene, 0.36 mg of alpha-carotene, and trace amounts of other carotenoids in vegetable oil | Human subject/ oral aplicated—22 patients, | ↑ MED ↑ serum beta-carotene levels ↑ inhibition of serum lipid peroxidation | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskwa, J.; Bronikowska, M.; Socha, K.; Markiewicz-Żukowska, R. Vegetable as a Source of Bioactive Compounds with Photoprotective Properties: Implication in the Aging Process. Nutrients 2023, 15, 3594. https://doi.org/10.3390/nu15163594
Moskwa J, Bronikowska M, Socha K, Markiewicz-Żukowska R. Vegetable as a Source of Bioactive Compounds with Photoprotective Properties: Implication in the Aging Process. Nutrients. 2023; 15(16):3594. https://doi.org/10.3390/nu15163594
Chicago/Turabian StyleMoskwa, Justyna, Monika Bronikowska, Katarzyna Socha, and Renata Markiewicz-Żukowska. 2023. "Vegetable as a Source of Bioactive Compounds with Photoprotective Properties: Implication in the Aging Process" Nutrients 15, no. 16: 3594. https://doi.org/10.3390/nu15163594