In Vitro Antiproliferative Apoptosis Induction and Cell Cycle Arrest Potential of Saudi Sidr Honey against Colorectal Cancer
Abstract
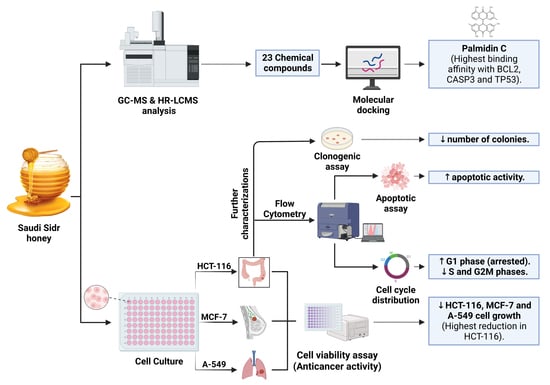
:1. Introduction
2. Materials and Methods
2.1. Collection of Honey Sample
2.2. Phytochemical Analysis of Saudi Sidr Honey
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Colony Formation Assay
2.6. Annexin V–PI Apoptosis Assay
2.7. Cell Cycle Analysis
2.8. Molecular Docking Analysis
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Composition of Saudi Sidr Honey
3.2. Anticancer Activity of Saudi Sidr Honey
3.3. Effect of Saudi Sidr Honey on Colony Formation of HCT-116 Cells
3.4. Apoptosis Induction by Saudi Sidr Honey
3.5. Saudi Sidr Honey Induces G1 Cell Cycle Arrest
3.6. Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Bhat, A.A.; Shakeel, A.; Rafiq, S.; Farooq, I.; Malik, A.Q.; Alghuthami, M.E.; Alharthi, S.; Qanash, H.; Alharthy, S.A. Juglans regia Linn.: A Natural Repository of Vital Phytochemical and Pharmacological Compounds. Life 2023, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Lotfollahzadeh, S.R.-B.A.; Cagir, B. Colon Cancer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470380/ (accessed on 10 January 2023).
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. New Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef]
- Schulmann, K.; Reiser, M.; Schmiegel, W. Colonic cancer and polyps. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 91–114. [Google Scholar] [CrossRef]
- Cooper, G.; Adams, K. The Cell: A Molecular Approach. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9963/ (accessed on 10 January 2023).
- Qanash, H.; Bazaid, A.S.; Alharazi, T.; Barnawi, H.; Alotaibi, K.; Shater, A.-R.M.; Abdelghany, T.M. Bioenvironmental applications of myco-created bioactive zinc oxide nanoparticle-doped selenium oxide nanoparticles. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Wiman, K.G.; Zhivotovsky, B. Understanding cell cycle and cell death regulation provides novel weapons against human diseases. J. Intern. Med. 2017, 281, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef]
- Imran, A.; Qamar, H.Y.; Ali, Q.; Naeem, H.; Riaz, M.; Amin, S.; Kanwal, N.; Ali, F.; Sabar, M.F.; Nasir, I.A. Role of Molecular Biology in Cancer Treatment: A Review Article. Iran. J. Public Health 2017, 46, 1475–1485. [Google Scholar]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.N.; Adnan, M.; Alreshidi, M.M.; Saeed, M.; Patel, M. Evaluation of Anticancer, Antibacterial and Antioxidant Properties of a Medicinally Treasured Fern Tectaria coadunata with its Phytoconstituents Analysis by HR-LCMS. Anticancer Agents Med. Chem. 2020, 20, 1845–1856. [Google Scholar] [CrossRef]
- Bhatt, M.; Patel, M.; Adnan, M.; Reddy, M.N. Anti-Metastatic Effects of Lupeol via the Inhibition of MAPK/ERK Pathway in Lung Cancer. Anticancer Agents Med. Chem. 2021, 21, 201–206. [Google Scholar] [CrossRef]
- Zaman, A.; Khan, M.S.; Akter, L.; Syeed, S.H.; Akter, J.; Al Mamun, A.; Alam, M.E.; Habib, M.A.; Jalil, M.A. Exploring new pharmacology and toxicological screening and safety evaluation of one widely used formulation of Nidrakar Bati from South Asia region. BMC Complement. Altern. Med. 2015, 15, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tafere, D.A. Chemical composition and uses of Honey: A Review. J. Food Sci. Nutr. Res. 2021, 4, 194–201. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Honey as a Potential Natural Anticancer Agent: A Review of Its Mechanisms. Evid. Based Complement. Altern. Med. 2013, 2013, 829070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abubakar, M.B.; Abdullah, W.Z.; Sulaiman, S.A.; Suen, A.B. A review of molecular mechanisms of the anti-leukemic effects of phenolic compounds in honey. Int. J. Mol. Sci. 2012, 13, 15054–15073. [Google Scholar] [CrossRef] [Green Version]
- Othman, N.H. Honey and cancer: Sustainable inverse relationship particularly for developing nations—A review. Evid. Based Complement. Altern. Med. 2012, 2012, 410406. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K.; Balaji, A.; Vellayappan, M.V.; Asokan, M.K.; Subramanian, A.P.; John, A.A.; Supriyanto, E.; Razak, S.I.; Marvibaigi, M. A review on antiproliferative and apoptotic activities of natural honey. Anticancer Agents Med. Chem. 2015, 15, 48–56. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Hussain, M.B.; Saeed, F.; Waheed, M.; Tufail, T. Phytochemistry, metabolism, and ethnomedical scenario of honey: A concurrent review. Int. J. Food Prop. 2017, 20, S254–S269. [Google Scholar] [CrossRef] [Green Version]
- Nikhat, S.; Fazil, M. History, phytochemistry, experimental pharmacology and clinical uses of honey: A comprehensive review with special reference to Unani medicine. J. Ethnopharmacol. 2022, 282, 114614. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yelin, A.; Kuntadi, K. Phytochemical identification of honey from several regions in Java and Sumbawa. AIP Conf. Proc. 2019, 2120, 080024. [Google Scholar] [CrossRef]
- Aldarhami, A.; Bazaid, A.S.; Qanash, H.; Ahmad, I.; Alshammari, F.H.; Alshammari, A.M.; Alshammari, A.H.; Aljanfawe, F.M.; Aldamiri, B.; Aldawood, E.; et al. Effects of Repeated in-vitro Exposure to Saudi Honey on Bacterial Resistance to Antibiotics and Biofilm Formation. Infect. Drug Resist. 2023, 16, 4273–4283. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Ansari, M.J. Biological and therapeutic roles of Saudi Arabian honey: A comparative review. J. King Saud Univ. Sci. 2021, 33, 101329. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Honey induces apoptosis in renal cell carcinoma. Pharmacogn. Mag. 2011, 7, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Jubri, Z.; Raja, J.; Nabilah, N.; Narayanan, N.; Aziz, M.A.; Karim, N.A.; Zurinah, W.; Ngah, W.S.W. Antiproliferative Activity and Apoptosis Induction by Gelam Honey on Liver Cancer Cell Line. Int. J. Appl. 2012, 2, 135–141. [Google Scholar]
- Badolato, M.; Carullo, G.; Cione, E.; Aiello, F.; Caroleo, M.C. From the hive: Honey, a novel weapon against cancer. Eur. J. Med. Chem. 2017, 142, 290–299. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Aldarhami, A.; Gattan, H.; Aljuhani, B. Saudi Honey: A Promising Therapeutic Agent for Treating Wound Infections. Cureus 2021, 13, e18882. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Aldarhami, A.; Patel, M.; Adnan, M.; Hamdi, A.; Snoussi, M.; Qanash, H.; Imam, M.; Monjed, M.K.; Khateb, A.M. The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: Phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities. Pharmaceuticals 2022, 15, 1212. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; Abdelghany, T.M. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Elasbali, A.M.; Al-Soud, W.A.; Al-Oanzi, Z.H.; Qanash, H.; Alharbi, B.; Binsaleh, N.K.; Alreshidi, M.; Patel, M.; Adnan, M. Cytotoxic Activity, Cell Cycle Inhibition, and Apoptosis-Inducing Potential of Athyrium hohenackerianum (Lady Fern) with Its Phytochemical Profiling. Evid. Based Complement. Altern. Med. 2022, 2022, 2055773. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Abdelghany, T.M. Pharmacological Evaluation of Acacia nilotica Flower Extract against Helicobacter pylori and Human Hepatocellular Carcinoma In Vitro and In Silico. J. Funct. Biomater. 2023, 14, 237. [Google Scholar] [CrossRef]
- Baskar, A.A.; Ignacimuthu, S.; Paulraj, G.M.; Al Numair, K.S. Chemopreventive potential of beta-Sitosterol in experimental colon cancer model—An in vitro and In vivo study. BMC Complement. Altern. Med. 2010, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Pozarowski, P.; Darzynkiewicz, Z. Analysis of cell cycle by flow cytometry. Methods Mol. Biol. 2004, 281, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for ligand-receptor docking. In Current Protocols in Bioinformatics; Wiley Online Library: Hoboken, NJ, USA, 2008; Chapter 8, Unit 8.14. [Google Scholar] [CrossRef]
- Albu, A.; Radu-Rusu, C.-G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality Assessment of Raw Honey Issued from Eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Al Guthami, F.M.; Ramadan, M.F.A.; Al Gethami, A.F.M.; Craig, A.M.; Serrano, S. Characterization of Sidr (Ziziphus spp.) Honey from Different Geographical Origins. Appl. Sci. 2022, 12, 9295. [Google Scholar] [CrossRef]
- Ismail, Z.B.; Alshehabat, M.A.; Hananeh, W.M.; Daradka, M.H.; Ali, J.H.; El-Najjar, E.K.M. Recent advances in topical wound healing products with special reference to honey: A review. Res. Opin. Anim. Vet. Sci. 2015, 5, 76–83. [Google Scholar]
- Alandejani, T.; Marsan, J.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Head Neck Surg. 2009, 141, 114–118. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waheed, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and cancer: A mechanistic review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Bouali, N.; Hamadou, W.S.; Badraoui, R.; Lajimi, R.H.; Hamdi, A.; Alreshidi, M.; Adnan, M.; Soua, Z.; Siddiqui, A.J.; Noumi, E.; et al. Phytochemical Composition, Antioxidant, and Anticancer Activities of Sidr Honey: In Vitro and In Silico Computational Investigation. Life 2023, 13, 35. [Google Scholar] [CrossRef]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sinsica 2005, 37, 719–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tor, Y.S.; Yazan, L.S.; Foo, J.B.; Armania, N.; Cheah, Y.K.; Abdullah, R.; Imam, M.U.; Ismail, N.; Ismail, M. Induction of apoptosis through oxidative stress-related pathways in MCF-7, human breast cancer cells, by ethyl acetate extract of Dillenia suffruticosa. BMC Complement. Altern. Med. 2014, 14, 55. [Google Scholar] [CrossRef] [Green Version]
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol. Oncol. 2012, 6, 155–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.Q.; Jaganath, I.B.; Sekaran, S.D. Phyllanthus spp. induces selective growth inhibition of PC-3 and MeWo human cancer cells through modulation of cell cycle and induction of apoptosis. PLoS ONE 2010, 5, e12644. [Google Scholar] [CrossRef] [Green Version]
- Manal Mohamed Elhassan, T.; Siddig Ibrahim, A.; Rashad, E.; Bassem, Y.S.; Mahmood Ameen, A.; Saif Eldeen, B.; Husham, E.; Eldaw, M. Effectiveness of Sidr Honey on the prevention of ethanol-induced gatroulcerogenesis: Role of antioxidant and antiapoptotic mechanism. Pharmacogn. J. 2015, 7, 157–164. [Google Scholar]
- Ghramh, H.A.; Ibrahim, E.H.; Kilany, M. Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci. Nutr. 2020, 8, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Djebli, N.; Mustafa, M.R.; Keskin, M.; Kolayli, S. Anti-Ulcerogenic and Cytoprotective Effects of Saharian (Sidr) Honey from Algeria. Comb. Chem. High Throughput Screen. 2021, 24, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.P.; Kumar, A.; Maurya, S.; Singh, A.K.; Sharma, A.K.; Kumar, S. Bulbine frutescens Phytochemicals as a Promising Anti-cancer Drug Discovery Source: A Computational Study. In Phytochemistry: An In-Silico and In-Vitro Update: Advances in Phytochemical Research; Kumar, S., Egbuna, C., Eds.; Springer: Singapore, 2019; pp. 491–510. [Google Scholar]









| Compounds | Structure | RT (min) |
|---|---|---|
| Glyceraldehyde |  | 6.526 |
| Butanedioic acid |  | 17.681 |
| Tetrahydro-4H-pyran-4-ol |  | 19.335 |
| 1-Cyclohexylimidazolidin-2-one |  | 23.937 |
| 3-butyl-3-methyl-1-Cyclohexanone |  | 25.170 |
| 4-Butyl-3-methoxy-2-cyclopenten-1-one |  | 25.284 |
| 2,2,3,3-Tetramethylcyclopropanecarboxylic acid |  | 29.099 |
| 3,5-Dihydroxy-2-(3-methylbut-2-en-1-yl) |  | 29.709 |
| Compounds | Mode of Analysis | Structure | RT (min) |
|---|---|---|---|
| Gerberinol | Positive |  | 1.014 |
| Osmaronin | Positive |  | 1.393 |
| Retronecine | Positive |  | 1.469 |
| N-(1-Deoxy-1-fructosyl) phenylalanine | Positive |  | 1.971 |
| C16 Sphinganine | Positive |  | 12.085 |
| 6-Hydroxypentadecanedioic acid | Positive |  | 12.788 |
| Sphinganine | Positive |  | 13.77 |
| Oleamide | Positive |  | 20.347 |
| Hamamelose | Negative |  | 1.236 |
| 2-O-b-D-Galactopyranosyl-D-xylose | Negative |  | 1.345 |
| 2,5-Didehydro-D-gluconate | Negative |  | 1.423 |
| Sarmentosin epoxide | Negative |  | 1.446 |
| Hellicoside | Negative |  | 7.818 |
| Palmidin C | Negative |  | 17.62 |
| 2-O-a-L-Fucopyranosyl-galactose | Negative |  | 21.505 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Patel, M.; Althomali, O.W.; Sheeha, B.B. In Vitro Antiproliferative Apoptosis Induction and Cell Cycle Arrest Potential of Saudi Sidr Honey against Colorectal Cancer. Nutrients 2023, 15, 3448. https://doi.org/10.3390/nu15153448
Qanash H, Bazaid AS, Binsaleh NK, Patel M, Althomali OW, Sheeha BB. In Vitro Antiproliferative Apoptosis Induction and Cell Cycle Arrest Potential of Saudi Sidr Honey against Colorectal Cancer. Nutrients. 2023; 15(15):3448. https://doi.org/10.3390/nu15153448
Chicago/Turabian StyleQanash, Husam, Abdulrahman S. Bazaid, Naif K. Binsaleh, Mitesh Patel, Omar W. Althomali, and Bodor Bin Sheeha. 2023. "In Vitro Antiproliferative Apoptosis Induction and Cell Cycle Arrest Potential of Saudi Sidr Honey against Colorectal Cancer" Nutrients 15, no. 15: 3448. https://doi.org/10.3390/nu15153448