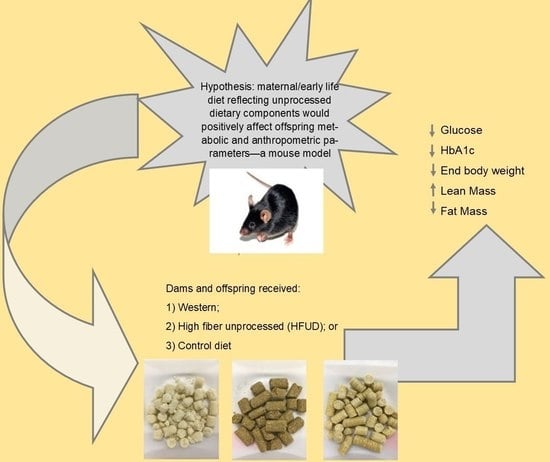
The Effects of a Western Diet vs. a High-Fiber Unprocessed Diet on Health Outcomes in Mice Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Procedure
- (1)
- Group 1 dams (n = 3) switched from the control diet to a high-fiber, low-sugar, high-monounsaturated and -polyunsaturated-fat diet (HFUD);
- (2)
- Group 2 dams (n = 3) switched from the control diet to a low-fiber, high-sugar, high-saturated-fat diet (WD);
- (3)
- Group 3 dams (n = 3) remained on the control diet [CD].
2.3. Diet Intervention
2.3.1. High-Fiber Unprocessed Diet (HFUD)
2.3.2. Western Diet (WD)
2.3.3. Control Diet
2.4. Measures
2.4.1. Body Weight and Composition
2.4.2. Biochemical Measures
2.5. Statistical Analysis
3. Results
3.1. Food Intake
3.2. Body Weight and Composition
3.3. Biochemical Assay
4. Discussion
5. Conclusions
Recommendations for Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia Drives Diet-Induced Obesity Independently of Brain Insulin Production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, T.; Kahn, C.R.; Accili, D. Insulin receptor knockout mice. Annu. Rev. Physiol. 2003, 65, 313–332. [Google Scholar] [CrossRef]
- Catalano, P.M. Obesity, insulin resistance, and pregnancy outcome. Reproduction 2010, 140, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Reichelt, A.C.; Lemieux, C.A.; Oren, P.L.; Ashmita, S.; Bussey, T.J.; Saksida, L.M. Age-dependent and region-specific alteration of parvalbumin neurons, perineuronal nets and microglia in the mouse prefrontal cortex and hippocampus following obesogenic diet consumption. Sci. Rep. (Nat. Publ. Group) 2021, 11, 5593. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.A.; DiPatrizio, N.V. Impact of maternal western diet-induced obesity on offspring mortality and peripheral endocannabinoid system in mice. PLoS ONE 2018, 13, e0205021. [Google Scholar] [CrossRef]
- Massiera, F.; Barbry, P.; Guesnet, P.; Joly, A.; Luquet, S.; Moreilhon-Brest, C.; Mohsen-Kanson, T.; Amri, E.-Z.; Ailhaud, G. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J. Lipid Res. 2010, 51, 2352–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Vedova, M.C.; Muñoz, M.D.; Santillan, L.D.; Plateo-Pignatari, M.G.; Germanó, M.J.; Tosi, M.E.R.; Garcia, S.; Gomez, N.N.; Fornes, M.W.; Mejiba, S.E.G.; et al. A Mouse Model of Diet-Induced Obesity Resembling Most Features of Human Metabolic Syndrome. Nutr. Metab. Insights 2016, 9, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Ribaroff, G.A.; Wastnedge, E.; Drake, A.J.; Sharpe, R.M.; Chambers, T.J.G. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: A meta-regression analysis. Obes. Rev. 2017, 18, 673–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Han, X.-F.; Fang, Z.-F.; Che, L.-Q.; Wu, D.; Wu, X.-Q.; Wu, C.-M. The beneficial effect of fiber supplementation in high- or low-fat diets on fetal development and antioxidant defense capacity in the rat. Eur. J. Nutr. 2012, 51, 19–27. [Google Scholar] [CrossRef]
- Taylor, P.D.; Poston, L. Developmental programming of obesity in mammals. Exp. Physiol. 2007, 92, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, R.M.; Osmond, C.; Phillips, D.I.W.; Godfrey, K.M. Maternal BMI, Parity, and Pregnancy Weight Gain: Influences on Offspring Adiposity in Young Adulthood. J. Clin. Endocrinol. Metab. 2010, 95, 5365–5369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.S.; Wang, M.C.; Freaney, P.M.; Perak, A.M.; Carnethon, M.R.; Kandula, N.R.; Gunderson, E.P.; Bullard, K.M.; Grobman, W.A.; O’brien, M.J.; et al. Trends in Gestational Diabetes at First Live Birth by Race and Ethnicity in the US, 2011-2019. JAMA 2021, 326, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Blysniuk, N.; Visram, A.; Lam, K.T. Gestational diabetes mellitus: A (nearly) perfect mouse model. J. Physiol. 2019, 597, 4689–4690. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015; Available online: http://www.diabetesatlas.org (accessed on 7 March 2023).
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; Castro, I.R.R.; Cannon, G. A new classification of foods based on the extent and purpose of food processing. Cadernos de Saúde Pública 2010, 26, 2039–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, M.A.; Baker, P.I. Ultra-processed food and adverse health outcomes. Br. Med. J. 2019, 365, l2289. [Google Scholar] [CrossRef]
- Satterfield, D.; DeBruyn, L.; Santos, M.; Alonso, L.; Frank, M. Health Promotion and Diabetes Prevention in American Indian and Alaska Native Communities—Traditional Foods Project, 2008–2014. MMWR Suppl. 2016, 65, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.M.; Pehrsson, P.R.; Agnew, W.W.; Scheett, A.J.; Follett, J.R.; Lukaski, H.C.; Patterson, K.Y. Nutrient composition of selected traditional United States Northern Plains Native American plant foods. J. Food Compost. Anal. 2014, 34, 136–152. [Google Scholar] [CrossRef] [Green Version]
- Conti, K. Nutrition Status of American Indian Adults and Impending Needs in View of the Strong Heart Dietary Study. J. Am. Diet Assoc. 2008, 108, 781–784. [Google Scholar] [CrossRef]
- Bersamin, A.; Luick, B.R.; King, I.B.; Stern, J.S.; Zidenberg-Cherr, S. Westernizing Diets Influence Fat Intake, Red Blood Cell Fatty Acid Composition, and Health in Remote Alaskan Native Communities in the Center for Alaska Native Health Study. J. Am. Diet Assoc. 2008, 108, 266–273. [Google Scholar] [CrossRef]
- Damman, S.; Eide, W.B.; Kuhnlein, H.V. Indigenous peoples’ nutrition transition in a right to food perspective. Food Policy 2008, 33, 135–155. [Google Scholar] [CrossRef]
- Kuhnlein, H.; Varthet, V.; Farren, A.; Falahi, E.; Leggee, D.; Receveur, O.; Berti, P. Vitamins A, D, and E, in Canadian Arctic traditional food and adult diets. J. Food. Comp. Anal. 2006, 19, 495–506. [Google Scholar] [CrossRef]
- Schulz, L.O.; Bennette, P.H.; Ravussen, E.; Kidd, J.R.; Kidd, K.K.; Esparza, J.; Valencia, M.E. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care 2006, 1866–1871. [Google Scholar] [CrossRef] [Green Version]
- Baldes, J.E. Cultural Plant Biodiversity in Relict Wallow-Like Depressions on the Wind River Indian Reservation, Wyoming, & Tribal Bison Restoration and Policy. Master’s Thesis, Montana State University, Bozeman, MT, USA, April 2016. [Google Scholar]
- Williams, D.E.; Knowler, W.C.; Smith, C.J.; Hanson, R.L.; Roumain, J.; Saremi, A.; Kriska, A.M.; Bennett, P.H.; Nelson, R.G. The Effect of Indian or Anglo Dietary Preference on the Incidence of Diabetes in Pima Indians. Diabetes Care 2001, 24, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeds, J.; Mansuri, S.; Mamakeesick, M.; Harris, S.B.; Zinman, B.; Gittelsohn, J.; Wolever, T.M.; Connelly, P.W.; Hanley, A. Dietary Patterns and Type 2 Diabetes Mellitus in a First Nations Community. Can. J. Diabetes 2016, 40, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Peltonen, M.; Eriksson, J.G.; Louheranta, A.; Fogelholm, M.; Uusitupa, M.; Tuomilehto, J. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: The Finnish Diabetes Prevention Study. Diabetologia 2006, 49, 912–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, T.T.; Hu, F.B.; Pereira, M.A.; Liu, S.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Whole-grain intake and the risk of type 2 diabetes: A prospective study in men. Am. J. Clin. Nutr. 2002, 76, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Moses, R.G.; Barker, M.; Winter, M.; Petocz, P.; Brand-Miller, J.C. Can a Low-Glycemic Index Diet Reduce the Need for Insulin in Gestational Diabetes Mellitus?: A randomized trial. Diabetes Care 2009, 32, 996–1000. [Google Scholar] [CrossRef] [Green Version]
- Kundi, Z.M.; Lee, J.C.; Pihlajamäki, J.; Chan, C.B.; Leung, K.S.; So, S.S.Y.; Nordlund, E.; Kolehmainen, M.; El-Nezami, H. Dietary Fiber from Oat and Rye Brans Ameliorate Western Diet–Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism. Mol. Nutr. Food Res. 2021, 65, 1900580. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, M.A.; Jacobs, D.R., Jr.; Pins, J.J.; Raatz, S.K.; Gross, M.D.; Slavin, J.L.; Seaquist, E.R. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am. J. Clin. Nutr. 2002, 75, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary Fiber Intake, Dietary Glycemic Load, and the Risk for Gestational Diabetes Mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef] [Green Version]
- Huishi, T.; Thompson, J.; Jiang, P. Maternal High-Fiber Diet Protects Offspring against Type 2 Diabetes. Nutrients 2021, 13, 94. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic Acid Prevents and Reverses Insulin Resistance in High-Fat Diet-Induced Obese Mice via Modulation of Adipose Tissue Inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, R.J.; Bilodeau, N.M.; Gordon, K.; Davis, A.D.; Stearns, J.C.; Cranmer-Byng, M.; Gasparelli, K.; Hill, L.D.; Anand, S.S. Entsisewata’karí:teke (You Will Be Healthy Again): Clinical Outcomes of Returning to a Traditional Haudenosaunee Diet. Int. J. Indig. Health 2021, 16, 82–119. [Google Scholar] [CrossRef]
- Ryman, T.; Boyer, B.; Hopkins, S.; Philip, J.; Beresford, S.; Thompson, B.; Heagerty, P.; Pomeroy, J.; Thummel, K.; Austin, M. Associations between diet and cardiometabolic risk among Yup’ik Alaska Native people using food frequency questionnaire dietary patterns. Nutr. Metab. Cardiovas. Dis. 2015, 25, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Gittelsohn, J.; Wolever, T.M.S.; Harris, S.B.; Harris-Giraldo, R.; Hanley, A.J.G.; Zinman, B. Specific Patterns of Food Consumption and Preparation Are Associated with Diabetes and Obesity in a Native Canadian Community. J. Nutr. 1998, 128, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E255–E266. [Google Scholar] [CrossRef]
- Whittingham, D.G.; Wood, M.J. Reproductive Physiology, Chapter 9. In The Mouse in Biomedical Research; American College of Laboratory Animal Medicine: New York, NY, USA, 1983; pp. 137–164. [Google Scholar]
- Bailoo, J.D.; Voelkl, B.; Varholick, J.; Novak, J.; Murphy, E.; Rosso, M.; Palme, R.; Würbel, H. Effects of weaning age and housing conditions on phenotypic differences in mice. Sci. Rep. 2020, 10, 11684. [Google Scholar] [CrossRef] [PubMed]
- Yusifov, A.; Chhatre, V.E.; Koplin, E.K.; Wilson, C.E.; Schmitt, E.E.; Woulfe, K.C.; Bruns, D.R. Transcriptomic analysis of cardiac gene expression across the life course in male and female mice. Physiol. Rep. 2021, 9, e14940. [Google Scholar] [CrossRef]
- Shi, H.; Akunuru, S.; Bierman, J.C.; Hodge, K.M.; Mitchell, M.C.; Foster, M.T.; Seeley, R.J.; Reizes, O. Diet-induced Obese Mice are Leptin Insufficient after Weight Reduction. Obesity 2009, 17, 1702–1709. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, F.; Akhoondan, M.; Ehteshami, M.; Aghamohammadi, V.; Ghanei, N.; Mirmiran, P.; Rashidkhani, B. Maternal Dietary Patterns and Gestational Diabetes Risk: A Case-Control Study. J. Diabetes Res. 2017, 2017, 5173926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhnlein, H.; Receveur, O. Dietary Change and Traditional Food Systems of Indigenous Peoples. Annu. Rev. Nutr. 1996, 16, 417–442. [Google Scholar] [CrossRef]
- Park, S.; Hongu, N.; Daily, J.W. Native American foods: History, culture, and influence on modern diets. J. Ethnic. Foods 2016, 3, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Byrd-Bredbenner, C.; Berning, J.; Kelley, D.; Abbot, J.; Moe, G.; Beshgetoor, D. Wardlaw’s Perspectives in Nutrition, 12th ed.; McGraw Hill: New York, NY, USA, 2022. [Google Scholar]
- Yusifov, A.; Chhatre, V.E.; Zumo, J.M.; Cook, R.F.; McNair, B.D.; Schmitt, E.E.; Woulfe, K.C.; Bruns, D.R. Cardiac response to adrenergic stress differs by sex and across the lifespan. GeroScience 2021, 43, 1799–1813. [Google Scholar] [CrossRef]
- Burke, S.J.; Batdorf, H.M.; Burk, D.H.; Noland, R.C.; Eder, A.E.; Boulos, M.S.; Karlstad, M.D.; Collier, J.J. db/db Mice Exhibit Features of Human Type 2 Diabetes That Are Not Present in Weight-Matched C57BL/6J Mice Fed a Western Diet. J. Diabetes Res. 2017, 2017, 8503754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNair, B.D.; Marcello, N.A.; Smith, D.T.; Schmitt, E.E.; Bruns, D.R. Changes in Muscle Mass and Composition by Exercise and Hypoxia as Assessed by DEXA in Mice. Medicina 2020, 56, 446. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shirakawa, J.; Okuyama, T.; Yamazaki, S.; Kyohara, M.; Miyazawa, A.; Suzuki, T.; Hamada, M.; Terauchi, Y. Evaluation of the appropriateness of using glucometers for measuring the blood glucose levels in mice. Sci. Rep. 2016, 6, 25465. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, S.J.; Chen, S.-Y.; Brandon, A.E.; Salamoun, J.M.; Byrne, F.L.; Garcia, C.J.; Beretta, M.; Olzomer, E.M.; Shah, D.P.; Philp, A.M.; et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat. Commun. 2020, 11, 2397. [Google Scholar] [CrossRef]
- Aerts, L.; Van Assche, F.A. Animal evidence for the transgenerational development of diabetes mellitus. Int. J. Biochem. Cell Biol. 2006, 38, 894–903. [Google Scholar] [CrossRef] [PubMed]
- CDC. Diabetes-Manage Blood Sugar—All about Your A1c. Available online: https://www.cdc.gov/diabetes/managing/managing-blood-sugar/a1c.html (accessed on 19 May 2023).
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, K.; Fujita, H.; Seto, Y.; Kato, R. Relation between Stable Glycated Hemoglobin A1c and Plasma Glucose Levels in Diabetes-model Mice. Exp. Anim. 1997, 46, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korstanje, R.; Ryan, J.L.; Savage, H.S.; Lyons, B.L.; Kane, K.G.; Sukoff Rizzo, S.J. Continuous Glucose Monitoring in Female NOD Mice Reveals Daily Rhythms and a Negative Correlation with Body Temperature. Endocrinology 2017, 158, 2707–2712. [Google Scholar] [CrossRef]
- Han, B.G.; Hao, C.-M.; Tchekneva, E.E.; Wang, Y.-Y.; Lee, C.A.; Ebrahim, B.; Harris, R.C.; Kern, T.S.; Wasserman, D.H.; Breyer, M.D.; et al. Markers of glycemic control in the mouse: Comparisons of 6-h- and overnight-fasted blood glucoses to Hb A1c. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E981–E986. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.; Kiersgaard, M.; Sørensen, D.; Mikkelsen, L. Fasting of mice: A review. Lab. Anim. 2013, 47, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.A.; Keim, K.S. Gilmore AC. Impact of core and secondary foods on nutritional composition of diets in Native-American women. J. Am. Diet Assoc. 2005, 105, 413–419. [Google Scholar] [CrossRef]
- Welty, T.K.; Rhoades, D.A.; Yeh, F.; Lee, E.T.; Cowan, L.D.; Fabsitz, R.R.; Robbins, D.C.; Devereux, R.B.; Henderson, J.A.; Howard, B.V. Changes in Cardiovascular Disease Risk Factors among American Indians: The Strong Heart Study. Ann. Epidemiol. 2002, 12, 97–106. [Google Scholar] [CrossRef]
- Liese, A.D. Dietary Glycemic Index and Glycemic Load, Carbohydrate and Fiber Intake, and Measures of Insulin Sensitivity, Secretion, and Adiposity in the Insulin Resistance Atherosclerosis Study|Diabetes Care|American Diabetes Association. Available online: https://diabetesjournals.org/care/article/28/12/2832/22736/Dietary-Glycemic-Index-and-Glycemic-Load (accessed on 7 January 2022).
- CDC. The Insulin Resistance–Diabetes Connection. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/diabetes/basics/insulin-resistance.html (accessed on 18 January 2022).
- Kalyani, R.R.; Tra, Y.; Egan, J.M.; Ferrucci, L.; Brancati, F. Hyperglycemia is associated with relatively lower lean body mass in older adults. J. Nutr. Health Aging 2014, 18, 737–743. [Google Scholar] [CrossRef] [Green Version]
- González-Granillo, M.; Steffensen, K.R.; Granados, O.; Torres, N.; Korach-André, M.; Ortíz, V.; Aguilar-Salinas, C.; Jakobsson, T.; Díaz-Villaseñor, A.; Loza-Valdes, A.; et al. Soy protein isoflavones differentially regulate liver X receptor isoforms to modulate lipid metabolism and cholesterol transport in the liver and intestine in mice. Diabetologia 2012, 55, 2469–2478. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, M.; Uemura, H.; Sakai, T.; Katsuura-Kamano, S.; Yamaguchi, M.; Hiyoshi, M.; Arisawa, K. Inverse association between soya food consumption and insulin resistance in Japanese adults. Public Health Nutr. 2015, 18, 2031–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, T.; Kang, Y.; Xu, X.; Wang, B.; Du, M.; Zhu, M.J. Raspberry Supplementation Improves Insulin Signaling and Promotes Brown-Like Adipocyte Development in White Adipose Tissue of Obese Mice. Mol. Nutr. Food Res. 2018, 62, 1701035. [Google Scholar] [CrossRef] [PubMed]

| Concentration % WD | Concentration % HFUD | Concentration % CD | |
|---|---|---|---|
| Protein | 15.5 | 23 | 28.5 |
| Fiber (g) | 5 | 23.5 | 5.1 |
| Carbohydrates | 43.1 | 50 | 58 |
| Fat | 41.4 | 26 | 13.5 |
| Sucrose (g) | 34 | 0 | 3.7 |
| WD n = 8 | HFUD n = 10 | CD n = 7 | ||||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Final body weight | 20.92 | 3.29 | 18.84 | 1.89 | 19.3 | 2.33 |
| Change in fat mass | +3.5 | +2.56 | +0.2 | +3.01 | +0.57 | +2.76 |
| Change in fat-free mass | −0.12 | +2.3 | +2.6 | +3.24 | +2.14 | +3.13 |
| Final insulin * | 0.12 | 0.03 | 0.1 | 0.02 | 0.08 | 0.02 |
| Final HbA1c * | 1.47 | 0.22 | 1.27 | 0.19 | 2.18 | 0.57 |
| Final glucose | 191.12 | 38.61 | 126.6 | 24.84 | 157.44 | 11.86 |
| WD vs. HFUD | WD vs. CD | HFUD vs. CD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Final body weight | 2.08 | 0.69 | 0.02 | 2.46 | 0.79 | 0.01 | 0.37 | 0.76 | 0.87 |
| Change in fat mass | 3.3 | 1.25 | 0.04 | 4.0 | 1.44 | 0.03 | 0.7 | 1.38 | 0.87 |
| Change in fat-free mass | −2.7 | 1.2 | 0.08 | −3.4 | 1.38 | 0.06 | −0.7 | 1.32 | 0.85 |
| Final insulin | 0.02 | 0.01 | 0.18 | 0.04 | 0.01 | 0.03 | 0.01 | 0.01 | 0.50 |
| Final HbA1c | 0.20 | 0.22 | 0.63 | −0.72 | 0.23 | 0.02 | −0.93 | 0.23 | <0.01 |
| Final glucose | 64.5 | 13.2 | <0.01 | 34.6 | 15.3 | 0.09 | −29.9 | 14.6 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzl, E.; Schmitt, E.E.; Shearrer, G.; Keith, J.F. The Effects of a Western Diet vs. a High-Fiber Unprocessed Diet on Health Outcomes in Mice Offspring. Nutrients 2023, 15, 2858. https://doi.org/10.3390/nu15132858
Herzl E, Schmitt EE, Shearrer G, Keith JF. The Effects of a Western Diet vs. a High-Fiber Unprocessed Diet on Health Outcomes in Mice Offspring. Nutrients. 2023; 15(13):2858. https://doi.org/10.3390/nu15132858
Chicago/Turabian StyleHerzl, Elizabeth, Emily E. Schmitt, Grace Shearrer, and Jill F. Keith. 2023. "The Effects of a Western Diet vs. a High-Fiber Unprocessed Diet on Health Outcomes in Mice Offspring" Nutrients 15, no. 13: 2858. https://doi.org/10.3390/nu15132858