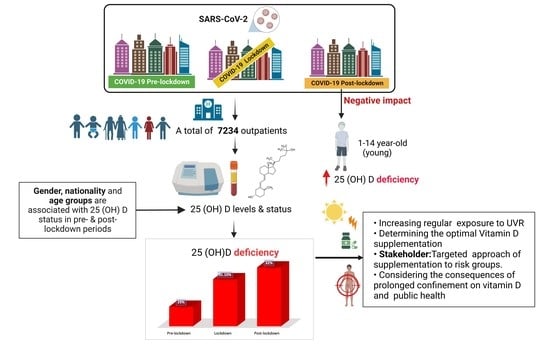
A Retrospective Chart Review Evaluating Changes in 25-Hydroxyvitamin D Levels among Patients Attending the University Healthcare Centre during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Participants
2.3. Inclusion and Exclusion Criteria
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Their Overall 25(OH) Vitamin D Status
3.2. The 25-(OH) D Serum Level during COVID-19 Pre-Lockdown, Lockdown and Post-Lockdown Periods
3.2.1. Changes in Serum Levels of 25 (OH) Vitamin D Stratified by Gender
3.2.2. 25 (OH) Vitamin D Status Stratified by Nationality
3.3. 25(OH) Vitamin D Status of Different Age Categories within the Three Periods
4. Discussion
Strengths and Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. Shedding New Light on the Role of the Sunshine Vitamin D for Skin Health: The LncRNA-Skin Cancer Connection. Exp. Dermatol. 2014, 23, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Stroud, M.L.; Stilgoe, S.; Stott, V.; Alhabian, O.; Salman, K. Vitamin D: A Review. Aust. J. Gen. Pract. 2008, 37, 1002. [Google Scholar]
- Crowe, F.L.; Jolly, K.; MacArthur, C.; Manaseki-Holland, S.; Gittoes, N.; Hewison, M.; Scragg, R.; Nirantharakumar, K. Trends in the Incidence of Testing for Vitamin D Deficiency in Primary Care in the UK: A Retrospective Analysis of the Health Improvement Network (THIN), 2005–2015. BMJ Open 2019, 9, e028355. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Binkley, N.; Ramamurthy, R.; Krueger, D. Low Vitamin D Status: Definition, Prevalence, Consequences, and Correction. Endocrinol. Metab. Clin. N. Am. 2010, 39, 287–301. [Google Scholar] [CrossRef]
- Karras, S.; Paschou, S.A.; Kandaraki, E.; Anagnostis, P.; Annweiler, C.; Tarlatzis, B.C.; Hollis, B.W.; Grant, W.B.; Goulis, D.G. Hypovitaminosis D in Pregnancy in the Mediterranean Region: A Systematic Review. Eur. J. Clin. Nutr. 2016, 70, 979–986. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and Vitamin D for Bone Health and Prevention of Autoimmune Diseases, Cancers, and Cardiovascular Disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- A Catharine Ross; To, C.; Al, E. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Aspell, N.; Laird, E.; Healy, M.; Shannon, T.; Lawlor, B.; O’Sullivan, M. The Prevalence and Determinants of Vitamin D Status in Community-Dwelling Older Adults: Results from the English Longitudinal Study of Ageing (ELSA). Nutrients 2019, 11, 1253. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and Bone Health; Potential Mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Gerkowicz, A.; Chyl-Surdacka, K.; Krasowska, D.; Chodorowska, G. The Role of Vitamin D in Non-Scarring Alopecia. Int. J. Mol. Sci. 2017, 18, 2653. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Gabaj, N.; Pavicic, T.; Vrtaric, A.; Milevoj Kopcinovic, L.; Herman Mahecic, D.; Bolanca, I.; Culej, J.; Miler, M.; Unic, A. In Sickness and in Health: Pivotal Role of Vitamin D. Biochem. Med. 2020, 30, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Vanderbruggen, N.; Matthys, F.; Laere, S.V.; Zeeuws, D.; Santermans, L.; den Ameele, S.V.; Crunelle, C.L. Self-Reported Alcohol, Tobacco, and Cannabis Use during COVID-19 Lockdown Measures: Results from a Web-Based Survey. Eur. Addict. Res. 2020, 26, 309–315. [Google Scholar] [CrossRef]
- Zachary, Z.; Brianna, F.; Brianna, L.; Garrett, P.; Jade, W.; Alyssa, D.; Mikayla, K. Self-Quarantine and Weight Gain Related Risk Factors during the COVID-19 Pandemic. Obes. Res. Clin. Pract. 2020, 14, 210–216. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ponzo, V.; Rosato, R.; Scumaci, E.; Goitre, I.; Benso, A.; Belcastro, S.; Crespi, C.; De Michieli, F.; Ghigo, E.; et al. Changes in Weight and Nutritional Habits in Adults with Obesity during the “Lockdown” Period Caused by the COVID-19 Virus Emergency. Nutrients 2020, 12, 2016. [Google Scholar] [CrossRef]
- Kifle, Z.D.; Woldeyohanins, A.E.; Asmare, B.; Atanaw, B.; Mesafint, T.; Adugna, M. Assessment of Lifestyle Changes during Coronavirus Disease 2019 Pandemic in Gondar Town, Northwest Ethiopia. PLoS ONE 2022, 17, e0264617. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D Deficiency Aggravates COVID-19: Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2020, 62, 1308–1316. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2019, 106, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Sarafin, K.; Durazo-Arvizu, R.; Tian, L.; Phinney, K.W.; Tai, S.; Camara, J.E.; Merkel, J.; Green, E.; Sempos, C.T.; Brooks, S.P. Standardizing 25-Hydroxyvitamin D Values from the Canadian Health Measures Survey. Am. J. Clin. Nutr. 2015, 102, 1044–1050. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National Estimates of Serum Total 25-Hydroxyvitamin D and Metabolite Concentrations Measured by Liquid Chromatography–Tandem Mass Spectrometry in the US Population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Al-Alyani, H.; Al-Turki, H.A.; Al-Essa, O.N.; Alani, F.M.; Sadat-Ali, M. Vitamin D Deficiency in Saudi Arabians: A Reality or Simply Hype: A Meta-Analysis (2008–2015). J Fam. Community Med. 2018, 25, 1–4. [Google Scholar]
- AlFaris, N.A.; AlKehayez, N.M.; AlMushawah, F.I.; AlNaeem, A.N.; AlAmri, N.D.; AlMudawah, E.S. Vitamin D Deficiency and Associated Risk Factors in Women from Riyadh, Saudi Arabia. Sci. Rep. 2019, 9, 20371. [Google Scholar] [CrossRef] [PubMed]
- Alsuwadia, A.O.; Farag, Y.M.; Al Sayyari, A.A.; Mousa, D.H.; Alhejaili, F.F.; Al-Harbi, A.S.; Housawi, A.A.; Mittal, B.V.; Singh, A.K. Prevalence of Vitamin D Deficiency in Saudi Adults. Saudi Med. J. 2013, 34, 814–818. [Google Scholar]
- Alzaheb, R.A.; Al-Amer, O. Prevalence and Predictors of Hypovitaminosis D among Female University Students in Tabuk, Saudi Arabia. Clin. Med. Insights Women’s Health 2017, 10, 1179562X1770239. [Google Scholar] [CrossRef] [PubMed]
- Ceolin, G.; Mano, G.P.R.; Hames, N.S.; da Antunes, L.C.; Brietzke, E.; Rieger, D.K.; Moreira, J.D. Vitamin D, Depressive Symptoms, and COVID-19 Pandemic. Front. Neurosci. 2021, 15, 670879. [Google Scholar] [CrossRef]
- De Winter, J.P.; de Winter, D.; Bollati, V.; Milani, G.P. A Safe Flight for Children through COVID-19 Disaster: Keeping Our Mind Open! Eur. J. Pediatr. 2020, 179, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi, M. The Preventive Strategies of COVID-19 Pandemic in Saudi Arabia. J. Microbiol. Immunol. Infect. 2020, 54, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Anil, I.; Alagha, O. The Impact of COVID-19 Lockdown on the Air Quality of Eastern Province, Saudi Arabia. Air Qual. Atmos. Health 2020, 14, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Saudi Arabia Imposes 24-Hour Curfew on Riyadh, Tabuk, Dammam, Dhahran, Hafouf, Jeddah, Taif, Qatif and Khobar, Interior Ministry Announces. The Official Saudi Press Agency. Published 6 April 2020. Available online: https://www.spa.gov.sa/viewfullstory.php?lang=en&newsid=2071013 (accessed on 7 April 2023).
- Mosca, C.; Colucci, A.; Savoia, F.; Calì, C.; Del Bene, M.; Ranucci, G.; Maglione, A.; Pepe, A.; Morelli, A.; Vajro, P.; et al. Vitamin D Levels in the Pre- and Post-COVID-19 Pandemic Periods and Related Confinement at Pediatric Age. Nutrients 2023, 15, 2089. [Google Scholar] [CrossRef]
- Nagakumar, P.; Chadwick, C.-L.; Bush, A.; Gupta, A. Collateral Impact of COVID-19: Why Should Children Continue to Suffer? Eur. J. Pediatr. 2021, 180, 1975–1979. [Google Scholar] [CrossRef]
- DeLuccia, R.; Clegg, D.; Sukumar, D. The Implications of Vitamin D Deficiency on COVID-19 for At-Risk Populations. Nutr. Rev. 2020, 79, 227–234. [Google Scholar] [CrossRef]
- Santaolalla, A.; Beckmann, K.; Kibaru, J.; Josephs, D.; Van Hemelrijck, M.; Irshad, S. Association between Vitamin D and Novel SARS-CoV-2 Respiratory Dysfunction—A Scoping Review of Current Evidence and Its Implication for COVID-19 Pandemic. Front. Physiol. 2020, 11, 564387. [Google Scholar] [CrossRef]
- Meoli, M.; Muggli, F.; Lava, S.A.G.; Bianchetti, M.G.; Agostoni, C.; Kocher, C.; Bührer, T.W.; Ciliberti, L.; Simonetti, G.D.; Milani, G.P. Vitamin D Status in Adolescents during COVID-19 Pandemic: A Cross-Sectional Comparative Study. Nutrients 2021, 13, 1467. [Google Scholar] [CrossRef]
- Farhat, K.H.; Arafa, M.A.; Rabah, D.M.; Amin, H.S.; Ibrahim, N.K. Vitamin D Status and Its Correlates in Saudi Male Population. BMC Public Health 2019, 19, 211. [Google Scholar] [CrossRef]
- Norton, J.C.; Politis, M.D.; Bimali, M.; Vyas, K.S.; Bircan, E.; Nembhard, W.N.; Amick, B.C.; Koturbash, I. Analysis of COVID-19 Pandemic on Supplement Usage and Its Combination with Self-Medication within the State of Arkansas. J. Diet. Suppl. 2022, 20, 171–198. [Google Scholar] [CrossRef]
- Shrestha, A.B.; Aryal, M.; Magar, J.R.; Shrestha, S.; Hossainy, L.; Rimti, F.H. The Scenario of Self-Medication Practices during the COVID-19 Pandemic; a Systematic Review. Ann. Med. Surg. 2022, 82, 104482. [Google Scholar] [CrossRef]
- Beyazgül, G.; Bağ, Ö.; Yurtseven, İ.; Coşkunol, F.; Başer, S.; Çiçek, D.; Kanberoğlu, G.İ.; Çelik, F.; Nalbantoğlu, Ö.; Özkan, B. How Did Vitamin D Levels of Children Change during COVID-19 Pandemic: A Comparison of Pre-Pandemic/Pandemic Periods due to Seasonal Differences. J. Clin. Res. Pediatr. Endocrinol. 2022, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Rustecka, A.; Maret, J.; Drab, A.; Leszczyńska, M.; Tomaszewska, A.; Lipińska-Opałka, A.; Będzichowska, A.; Kalicki, B.; Kubiak, J.Z. The Impact of COVID-19 Pandemic during 2020–2021 on the Vitamin D Serum Levels in the Paediatric Population in Warsaw, Poland. Nutrients 2021, 13, 1990. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Rustecka, A.; Lipińska-Opałka, A.; Piprek, R.P.; Kloc, M.; Kalicki, B.; Kubiak, J.Z. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front. Pharmacol. 2022, 13, 836738. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ke, H.-J.; Che, D.; Luo, S.-L.; Guo, Y.; Wu, J.-L. Effect of Pandemic-Related Confinement on Vitamin D Status among Children Aged 0–6 Years in Guangzhou, China: A Cross-Sectional Study. Risk Manag. Healthc. Policy 2020, 13, 2669–2675. [Google Scholar] [CrossRef]
- Gallagher, J.C. Vitamin D and Aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, A.; Ji, J.S. A Comparison Study of Vitamin D Deficiency among Older Adults in China and the United States. Sci. Rep. 2019, 9, 19713. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Camussi, E.; Piccinelli, C.; Senore, C.; Armaroli, P.; Ortale, A.; Garena, F.; Giordano, L. Did Social Isolation during the SARS-CoV-2 Epidemic Have an Impact on the Lifestyles of Citizens? L’isolamento Sociale Durante l’Epidemia Da SARS-CoV-2 Ha Avuto Un Impatto Sugli Stili Di Vita Dei Cittadini? Epidemiol. Prev. 2020, 44 (Suppl. S2), 353–362. [Google Scholar] [CrossRef]
- Nwosu, B.U.; Kum-Nji, P. Tobacco Smoke Exposure Is an Independent Predictor of Vitamin D Deficiency in US Children. PLoS ONE 2018, 13, e0205342. [Google Scholar] [CrossRef]
- Xiang, M.; Zhang, Z.; Kuwahara, K. Impact of COVID-19 Pandemic on Children and Adolescents’ Lifestyle Behavior Larger than Expected. Prog. Cardiovasc. Dis. 2020, 63, 531. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating Habits and Lifestyle Changes during COVID-19 Lockdown: An Italian Survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Soranna, D.; Zambra, G.; Zambon, A.; Invitti, C. Determinants of the Lifestyle Changes during COVID-19 Pandemic in the Residents of Northern Italy. Int. J. Environ. Res. Public Health 2020, 17, 6287. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Impact of Gender Difference on Vitamin D Status and Its Relationship with the Extent of Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Al-Horani, H.; Abu Dayyih, W.; Mallah, E.; Hamad, M.; Mima, M.; Awad, R.; Arafat, T. Nationality, Gender, Age, and Body Mass Index Influences on Vitamin D Concentration among Elderly Patients and Young Iraqi and Jordanian in Jordan. Biochem. Res. Int. 2016, 2016, 8920503. [Google Scholar] [CrossRef]
- Zhang, F.F.; Al Hooti, S.; Al Zenki, S.; Alomirah, H.; Jamil, K.M.; Rao, A.; Al Jahmah, N.; Saltzman, E.; Ausman, L.M. Vitamin D Deficiency Is Associated with High Prevalence of Diabetes in Kuwaiti Adults: Results from a National Survey. BMC Public Health 2016, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Botros, R.M.; Sabry, I.M.; Abdelbaky, R.S.; Eid, Y.M.; Nasr, M.S.; Hendawy, L.M. Vitamin D Deficiency among Healthy Egyptian Females. Endocrinol. Y Nutr. 2015, 62, 314–321. [Google Scholar] [CrossRef]
- Van der Meer, I.M.; Middelkoop, B.J.C.; Boeke, A.J.P.; Lips, P. Prevalence of Vitamin D Deficiency among Turkish, Moroccan, Indian and Sub-Sahara African Populations in Europe and Their Countries of Origin: An Overview. Osteoporos. Int. 2010, 22, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Khader, Y.S.; Batieha, A.; Jaddou, H.; Batieha, Z.; El-Khateeb, M.; Ajlouni, K. Relationship between 25-Hydroxyvitamin D and Metabolic Syndrome among Jordanian Adults. Nutr. Res. Pract. 2011, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.N.; Alkhenizan, A.H.; El Shaker, M.; Raef, H.; Gabr, A. Increasing Trends and Significance of Hypovitaminosis D: A Population-Based Study in the Kingdom of Saudi Arabia. Arch. Osteoporos. 2014, 9, 190. [Google Scholar] [CrossRef]
- Coşkun, S.; Şimşek, Ş.; Camkurt, M.A.; Çim, A.; Çelik, S.B. Association of Polymorphisms in the Vitamin D Receptor Gene and Serum 25-Hydroxyvitamin D Levels in Children with Autism Spectrum Disorder. Gene 2016, 588, 109–114. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B.; Benkusky, N.A.; Lee, S.M.; St. John, H.; Carlson, A.; Onal, M.; Shamsuzzaman, S. Genomic Determinants of Vitamin D-Regulated Gene Expression. Vitam. D Horm. 2016, 100, 21–44. [Google Scholar] [CrossRef]
- Sadat-Ali, M.; Al-Turki, H.; Azam, M.; Al-Elq, A. Genetic Influence on Circulating Vitamin D among Saudi Arabians. Saudi Med. J. 2016, 37, 996–1001. [Google Scholar] [CrossRef]
- Bu, F.-X.; Armas, L.; Lappe, J.; Zhou, Y.; Gao, G.; Wang, H.-W.; Recker, R.; Zhao, L.-J. Comprehensive Association Analysis of Nine Candidate Genes with Serum 25-Hydroxy Vitamin D Levels among Healthy Caucasian Subjects. Hum. Genet. 2010, 128, 549–556. [Google Scholar] [CrossRef]
- McGrath, J.J.; Saha, S.; Burne, T.H.J.; Eyles, D.W. A Systematic Review of the Association between Common Single Nucleotide Polymorphisms and 25-Hydroxyvitamin D Concentrations. J. Steroid Biochem. Mol. Biol. 2010, 121, 471–477. [Google Scholar] [CrossRef]
- ALbuloshi, T.; Kamel, A.M.; Spencer, J.P.E. Factors Associated with Low Vitamin D Status among Older Adults in Kuwait. Nutrients 2022, 14, 3342. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Fleet, J.C. THE GENETICS of OSTEOPOROSIS: Vitamin D Receptor Polymorphisms. Annu. Rev. Nutr. 1998, 18, 233–258. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Insufficiency/Deficiency in Gastrointestinal Disorders. J. Bone Miner. Res. 2007, 22 (Suppl. S2), V50–V54. [Google Scholar] [CrossRef]
- Gascon-Barré, M.; Demers, C.; Ghrab, O.; Theodoropoulos, C.; Lapointe, R.; Jones, G.; Valiquette, L.; Ménard, D. Expression of CYP27A, a Gene Encoding a Vitamin D-25 Hydroxylase in Human Liver and Kidney. Clin. Endocrinol. 2001, 54, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Alpalhão, M.; Filipe, P. SARS-CoV-2 Pandemic and Vitamin D Deficiency—A Double Trouble. Photodermatol. Photoimmunol. Photomed. 2020, 36, 412–413. [Google Scholar] [CrossRef]

| 25 (OH) D Status (ng/mL) | |||||
|---|---|---|---|---|---|
| Total | Sufficient | Insufficient | Deficient | ||
| n (%) | ≥30 ng/mL | 21–29 | 0–20 | ||
| Age | (1–105) | 7234 (100%) | 2553 (35.4%) | 2440(33.8%) | 2217(30.7%) |
| Categories | 1–14 | 533 (7.4%) | |||
| 15–18 | 310 (4.3%) | ||||
| 19–35 | 3131 (43.3%) | ||||
| 36–47 | 1508 (20.8%) | ||||
| 48–64 | 1382 (19.1%) | ||||
| 65–105 | 370 (5.1%) | ||||
| Mean (age) ± SD | 34.7 ± 16.8 | ||||
| Gender | |||||
| Male | 5302 (73.3%) | ||||
| Female | 1932 (26.7%) | ||||
| Nationality | |||||
| Saudi | 5461 (75.5%) | ||||
| Non-Saudi | 1773 (24.5%) | ||||
| 25 (OH)D Status Frequency n (%) | Sufficient (≥30 ng/mL) n = 2553 (35.3%) | Insufficient (21–29 ng/mL) n = 2440 (33.8%) | Deficient (0–20 ng/mL) n = 2217 (30.7%) | 25 (OH) D Concentration (Mean ± SD, ng/mL) | |
|---|---|---|---|---|---|
| COVID-19 Periods | Pre-lockdown | 965 (33.5%) | 1077 (37.5%) | 833 (29%) | 27.6 ± 11.87 |
| Lockdown | 68 (34.7%) | 67 (34.2%) | 61 (31.1) | 27.4 ± 12.52 | |
| Post-lockdown | 1520 (36.7%) | 1296 (31.3%) | 1323 (32%) | 27.7 ± 13.84 | |
| p-Value | b: p < 0.001 | b: p < 0.001 | b: p < 0.001 | a: 0.729 |
| 25 (OH) Vitamin D Serum Concentration (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Sufficient | Insufficient | Deficient | Total | p-Value | |||
| Pre-lockdown | Female | % Within Gender | 678 (31.1%) | 793 (36.4%) | 710. (32.6%) | 2181 (100%) | 0.0000 |
| % of Total | 23.60% | 27.60% | 24.70% | 75.90% | |||
| Male | % Within Gender | 287 (41.4%) | 284 (40.9%) | 123 (17.7%) | 694 (100%) | ||
| % of Total | 10.00% | 9.90% | 4.30% | 24.10% | |||
| Total | % Within Gender | 965 (33.6%) | 1077 (37.5%) | 833 (29%) | 2875 (100%) | ||
| Lockdown | Female | % Within Gender | 43 (36.1%) | 42 (35.3%) | 34 (28.6%) | 119 (100%) | 0.6300 |
| % of Total | 21.90% | 21.40% | 17.30% | 60.70% | |||
| Male | % Within Gender | 25 (32.5%) | 25 (32.5%) | 27 (35.1%) | 77 (100%) | ||
| % of Total | 12.80% | 12.80% | 13.80% | 39.30% | |||
| Total | % Within Gender | 68 (34.7%) | 67 (34.2%) | 61 (31.1%) | 196 (100%) | ||
| Post-lockdown | Female | % Within Gender | 1095 (36.7%) | 903 (30.3%) | 982 (33%) | 2980 (100%) | 0.0350 |
| % of Total | 26.50% | 21.80% | 23.70% | 72.00% | |||
| Male | % Within Gender | 425 (36.7%) | 393 (33.9%) | 341 (29.4%) | 1159 (100%) | ||
| % of Total | 10.30% | 9.50% | 8.20% | 28.00% | |||
| Total | % Within Gender | 1520 (36.7%) | 1296 (31.3%) | 1323 (32%) | 4139 (100%) | ||
| Nationality | N (Frequency) | Mean of 25 (OH) D Concentrations | Std. Deviation | p-Value | ||
|---|---|---|---|---|---|---|
| Pre-lockdown | 25 (OH) D | Non-Saudi | 632 | 30.01 | 11.32 | 0.000 |
| Saudi | 2255 | 26.91 | 11.94 | |||
| Lockdown | 25 (OH) D | Non-Saudi | 69 | 28.58 | 12.85 | 0.319 |
| Saudi | 127 | 26.70 | 12.35 | |||
| Post-lockdown | 25 (OH) D | Non-Saudi | 1070 | 29.55 | 13.58 | 0.000 |
| Saudi | 3074 | 27.21 | 13.88 |
| 25 (OH) Vitamin D Status (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sufficient | Insufficient | Deficient | Total | p-Value | ||||
| Pre-lockdown | Nationality | Non-Saudi | ||||||
| % Within Nationality | 270 (42.9%) | 254 (40.3%) | 106 (16.8%) | 630 (100%) | 0.000 | |||
| % of Total | 9.40% | 8.80% | 3.70% | 21.90% | ||||
| Saudi | % Within Nationality | 695 (31%) | 823 (36.7%) | 727 (32.4%) | 2245 (100%) | |||
| % of Total | 24.20% | 28.60% | 25.30% | 78.10% | ||||
| Total | % Within Nationality | 965 (33.6%) | 1077 (37.5%) | 833 (29%) | 2875 (100%) | |||
| Lockdown | Nationality | Non-Saudi | 0.000 | |||||
| % Within Nationality | 25 (36.2%) | 25 (36.2%) | 19 (27.5%) | 69 (100%) | ||||
| % of Total | 12.80% | 12.80% | 9.70% | 35.20% | ||||
| Saudi | ||||||||
| % Within Nationality | 43 (33.9%) | 42 (33.1%) | 42 (33.1%) | 127 (100%) | ||||
| % of Total | 21.90% | 21.40% | 21.40% | 64.80% | ||||
| Total | % Within Nationality | 68 (34.7%) | 67 (34.2%) | 61 (31.1%) | 196 (100%) | |||
| Post-lockdown | Nationality | Non-Saudi | 0.000 | |||||
| % Within Nationality | 451 (42.1%) | 382 (35.7%) | 237 (22.1%) | 1070 (100%) | ||||
| % of Total | 10.90% | 9.20% | 5.70% | 25.90% | ||||
| Saudi | ||||||||
| % Within Nationality | 1069 (34.8%) | 914 (29.8%) | 1086 (35.4%) | 3069 (100%) | ||||
| % of Total | 25.80% | 22.10% | 26.20% | 74.10% | ||||
| Total | % Within Nationality | 1520 (36.7%) | 1296 (31.3%) | 1323 (32%) | 4139 (100%) | |||
| Means of 25 (OH) D Concentrations (ng/mL) | ||||
|---|---|---|---|---|
| Age Categories | N (Frequency) | Pre-Lockdown | Lockdown | Post-Lockdown |
| 1–14 | 226 | 32.75 | 23.93 | 28.27 |
| 15–18 | 99 | 23.76 | 22.58 | 25.19 |
| 19–35 | 1439 | 26.23 | 27.49 | 26.60 |
| 36–47 | 501 | 27.35 | 26.52 | 28.18 |
| 48–64 | 480 | 28.97 | 28.37 | 29.53 |
| 65–105 | 142 | 32.03 | 36.65 | 30.52 |
| Total | 2887 | 27.59 | 27.36 | 27.81 |
| Coefficients a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Periods | Unstandardized Coefficients | Standardized Coefficients | 95.0% Confidence Interval for B | ||||||
| B | Std. Error | Beta | t | Sig. | Lower Bound | Upper Bound | |||
| Pre-lockdown | 1 | (Constant) | 28.02 | 0.68 | 40.91 | 0.00 | 26.67 | 29.36 | |
| Age | 0.03 | 0.01 | 0.04 | 2.31 | 0.02 | 0.01 | 0.06 | ||
| Nationality | −2.65 | 0.53 | −0.09 | −4.95 | 0.00 | −3.70 | −1.60 | ||
| Gender | 2.69 | 0.52 | 0.10 | 5.22 | 0.00 | 1.68 | 3.70 | ||
| Lockdown | 1 | (Constant) | 24.26 | 2.54 | 9.54 | 0.00 | 19.24 | 29.28 | |
| Age | 0.12 | 0.05 | 0.17 | 2.31 | 0.02 | 0.02 | 0.22 | ||
| Nationality | −1.76 | 1.86 | −0.07 | −0.95 | 0.34 | −5.44 | 1.91 | ||
| Gender | −1.42 | 1.90 | −0.06 | −0.75 | 0.45 | −5.17 | 2.32 | ||
| Post-lockdown | 1 | (Constant) | 27.22 | 0.65 | 41.70 | 0.00 | 25.94 | 28.50 | |
| Age | 0.07 | 0.01 | 0.09 | 5.48 | 0.00 | 0.05 | 0.10 | ||
| Nationality | −2.28 | 0.49 | −0.07 | −4.62 | 0.00 | −3.25 | −1.31 | ||
| Gender | −0.93 | 0.49 | −0.03 | −1.92 | 0.06 | −1.88 | 0.02 | ||
| Variables in the Equation | 95% Confidence Interval | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Periods | B | S.E. | Wald | df | Sig. | Odd Ratio | Lower | Upper | ||
| Prelockdown | Step 1 a | Gender | 0.447 | 0.09 | 24.695 | 1 | 0.000 | 1.563 | 1.311 | 1.864 |
| Constant | −0.8 | 0.046 | 296.099 | 1 | 0.000 | |||||
| Lockdown | Step 1 a | Gender | −0.16 | 0.309 | 0.277 | 1 | 0.599 | 0.850 | 0.463 | 1.558 |
| Constant | −0.57 | 0.191 | 8.908 | 1 | 0.003 | |||||
| Post-lockdown | Step 1 a | Gender | 0 | 0.072 | 0.002 | 1 | 0.964 | 0.997 | 0.866 | 1.147 |
| Constant | −0.54 | 0.038 | 204.355 | 1 | 0.000 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benameur, T.; Kaliyadan, F.; Saidi, N.; Porro, C. A Retrospective Chart Review Evaluating Changes in 25-Hydroxyvitamin D Levels among Patients Attending the University Healthcare Centre during the COVID-19 Pandemic. Nutrients 2023, 15, 2345. https://doi.org/10.3390/nu15102345
Benameur T, Kaliyadan F, Saidi N, Porro C. A Retrospective Chart Review Evaluating Changes in 25-Hydroxyvitamin D Levels among Patients Attending the University Healthcare Centre during the COVID-19 Pandemic. Nutrients. 2023; 15(10):2345. https://doi.org/10.3390/nu15102345
Chicago/Turabian StyleBenameur, Tarek, Feroze Kaliyadan, Neji Saidi, and Chiara Porro. 2023. "A Retrospective Chart Review Evaluating Changes in 25-Hydroxyvitamin D Levels among Patients Attending the University Healthcare Centre during the COVID-19 Pandemic" Nutrients 15, no. 10: 2345. https://doi.org/10.3390/nu15102345