Associations of Serum 25(OH)D, PTH, and β-CTX Levels with All-Cause Mortality in Chinese Community-Dwelling Centenarians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Covariates
2.3. Statistical Analysis
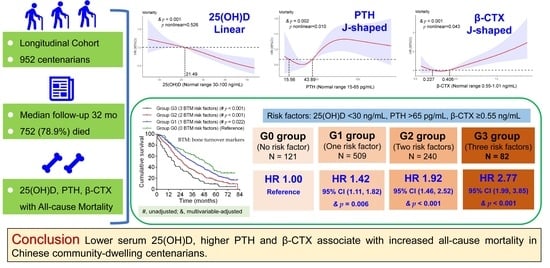
3. Results
3.1. Baseline Characteristics and Follow-Up
3.2. Associations of 25(OH)D, PTH, and β-CTX with All-Cause Mortality
3.3. Combined Effects of 25(OH)D, PTH and β-CTX on All-Cause Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferri, E.; Casati, M.; Cesari, M.; Vitale, G.; Arosio, B. Vitamin D in physiological and pathological aging: Lesson from centenarians. Rev. Endocr. Metab. Disord. 2019, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheater, G.; Elshahaly, M.; Tuck, S.P.; Datta, H.K.; van Laar, J.M. The clinical utility of bone marker measurements in osteoporosis. J. Transl. Med. 2013, 11, 201. [Google Scholar] [CrossRef] [Green Version]
- Michos, E.D.; Cainzos-Achirica, M.; Heravi, A.S.; Appel, L.J. Vitamin D, Calcium Supplements, and Implications for Cardiovascular Health: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; März, W.; Pilz, S. Vitamin D and Cardiovascular Disease: An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 2896. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef] [Green Version]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Passeri, G.; Vescovini, R.; Sansoni, P.; Galli, C.; Franceschi, C.; Passeri, M. Calcium metabolism and vitamin D in the extreme longevity. Exp. Gerontol. 2008, 43, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Xiong, S.; Ju, S.Y.; Zeng, Y.; Yan, L.L.; Yao, Y. Serum 25-Hydroxyvitamin D, Albumin, and Mortality Among Chinese Older Adults: A Population-based Longitudinal Study. J. Clin. Endocrinol. Metab. 2020, 105, 2762–2770. [Google Scholar] [CrossRef]
- Mao, C.; Li, F.R.; Yin, Z.X.; Lv, Y.B.; Luo, J.S.; Yuan, J.Q.; Mhungu, F.; Wang, J.N.; Shi, W.Y.; Zhou, J.H.; et al. Plasma 25-Hydroxyvitamin D Concentrations Are Inversely Associated with All-Cause Mortality among a Prospective Cohort of Chinese Adults Aged >/=80 Years. J. Nutr. 2019, 149, 1056–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samefors, M.; Östgren, C.J.; Mölstad, S.; Lannering, C.; Midlöv, P.; Tengblad, A. Vitamin D defi-ciency in elderly people in Swedish nursing homes is associated with increased mortality. Eur. J. Endocrinol. 2014, 170, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; Dobnig, H.; Tomaschitz, A.; Kienreich, K.; Meinitzer, A.; Friedl, C.; Wagner, D.; Piswang-er-Sölkner, C.; März, W.; Fahrleitner-Pammer, A. Low 25-hydroxyvitamin D is associated with in-creased mortality in female nursing home residents. J. Clin. Endocrinol. Metab. 2012, 97, E653–E657. [Google Scholar] [CrossRef] [Green Version]
- Granic, A.; Aspray, T.; Hill, T.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; von Zglinicki, T.; Kirkwood, T.B.; Mathers, J.C.; Jagger, C. 25-hydroxyvitamin D and increased all-cause mortality in very old women: The Newcastle 85+ study. J. Intern. Med. 2015, 277, 456–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formiga, F.; Ferrer, A.; Megido, M.J.; Boix, L.; Contra, A.; Pujol, R. Low serum vitamin D is not as-sociated with an increase in mortality in oldest old subjects: The Octabaix three-year follow-up study. Gerontology 2014, 60, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Nakamura, K.; Nishiwaki, T.; Ueno, K.; Hasegawa, M. Low body mass index and low serum albumin are predictive factors for short-term mortality in elderly Japanese requiring home care. Tohoku J. Exp. Med. 2010, 221, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Aucott, L.S.; McNeill, G. Nutritional status and subsequent all-cause mortality in men and women aged 75 years or over living in the community. Br. J. Nutr. 2007, 98, 593–599. [Google Scholar] [CrossRef] [Green Version]
- van Ballegooijen, A.J.; Reinders, I.; Visser, M.; Brouwer, I.A. Parathyroid hormone and cardiovascular disease events: A systematic review and meta-analysis of prospective studies. Am. Heart J. 2013, 165, 655–664. [Google Scholar] [CrossRef]
- Yang, B.; Lu, C.; Wu, Q.; Zhang, J.; Zhao, H.; Cao, Y. Parathyroid hormone, cardiovascular and all-cause mortality: A meta-analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 455, 154–160. [Google Scholar] [CrossRef]
- Barasch, E.; Gottdiener, J.S.; Aurigemma, G.; Kitzman, D.W.; Han, J.; Kop, W.J.; Tracy, R.P. The relationship between serum markers of collagen turnover and cardiovascular outcome in the elderly: The Cardiovascular Health Study. Circulation. Heart Fail. 2011, 4, 733–739. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Jarolim, P.; Scirica, B.M.; Braunwald, E.; Park, J.G.; Das, S.; Sabatine, M.S.; Morrow, D.A. Biomarker of Collagen Turnover (C-Terminal Telopeptide) and Prognosis in Patients With Non- ST -Elevation Acute Coronary Syndromes. J. Am. Heart Assoc. 2019, 8, e011444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerchbaum, E.; Schwetz, V.; Pilz, S.; Boehm, B.O.; März, W. Association of bone turnover markers with mortality in women referred to coronary angiography: The Ludwigshafen Risk and Cardio-vascular Health (LURIC) study. Osteoporos. Int. 2014, 25, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Schwetz, V.; Pilz, S.; Grammer, T.B.; Look, M.; Boehm, B.O.; Obermayer-Pietsch, B.; März, W. Association of bone turnover markers with mortality in men referred to coronary angiography. Osteoporos. Int. 2013, 24, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, Y.; Yao, Y.; Yang, S.; Li, J.; Liu, M.; Chen, X.; Wang, J.; Zhu, Q.; Li, X.; et al. Cohort Profile: The China Hainan Centenarian Cohort Study (CHCCS). Int. J. Epidemiol. 2018, 47, 694–695h. [Google Scholar] [CrossRef]
- Fu, S.; Ping, P.; Li, Y.; Li, B.; Zhao, Y.; Yao, Y.; Zhang, P. Centenarian longevity had inverse relationships with nutritional status and abdominal obesity and positive relationships with sex hormones and bone turnover in the oldest females. J. Transl. Med. 2021, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Mu-rad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Fu, S.; Zhang, H.; Li, N.; Zhu, Q.; Zhang, F.; Luan, F.; Zhao, Y.; He, Y. The prevalence of depressive symptoms in Chinese longevous persons and its correlation with vitamin D status. BMC Geriatr. 2018, 18, 198. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Fu, S.; Shi, Q.; Zhang, H.; Zhu, Q.; Zhang, F.; Luan, F.; Zhao, Y.; He, Y. Prevalence of functional dependence in Chinese centenarians and its relationship with serum vitamin D status. Clin. Interv. Aging 2018, 13, 2045–2053. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Fu, S.; Zhao, M.; Liu, D.; Zhao, Y.; Yao, Y. Associations Between Complement Components and Vitamin D and the Physical Activities of Daily Living Among a Longevous Population in Hainan, China. Front. Immunol. 2020, 11, 1543. [Google Scholar] [CrossRef]
- Sambrook, P.N.; Chen, J.S.; March, L.M.; Cameron, I.D.; Cumming, R.G.; Lord, S.R.; Schwarz, J.; Seibel, M.J. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin d status, bone mass, and renal function in the frail and very old: A cohort study. J. Clin. Endocrinol. Metab. 2004, 89, 5477–5481. [Google Scholar] [CrossRef]
- Björkman, M.P.; Sorva, A.J.; Tilvis, R.S. Elevated serum parathyroid hormone predicts impaired survival prognosis in a general aged population. Eur. J. Endocrinol. 2008, 158, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchebner, D.; Malmgren, L.; Christensson, A.; McGuigan, F.; Gerdhem, P.; Ridderstrale, M.; Akesson, K. Longitudinal Assessment of PTH in Community-Dwelling Older Women-Elevations Are Not Associated With Mortality. J. Endocr. Soc. 2017, 1, 615–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollerslev, J.; Sjöstedt, E.; Rejnmark, L. Cardiovascular consequences of parathyroid disorders in adults. Ann. d’Endocrinol. 2021, 82, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Toribio, R.E.; Kohn, C.W.; Capen, C.C.; Rosol, T.J. Parathyroid hormone (PTH) secretion, PTH mRNA and calcium-sensing receptor mRNA expression in equine parathyroid cells, and effects of interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha on equine parathyroid cell function. J. Mol. Endocrinol. 2003, 31, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Guise, T.A.; Garrett, I.R.; Bonewald, L.F.; Mundy, G.R. Interleukin-1 receptor antagonist inhibits the hypercalcemia mediated by interleukin-1. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1993, 8, 583–587. [Google Scholar] [CrossRef]
- Buckley, L.F.; Abbate, A. Interleukin-1 blockade in cardiovascular diseases: A clinical update. Eur. Heart J. 2018, 39, 2063–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bager, C.L.; Willumsen, N.; Christiansen, C.; Bay-Jensen, A.C.; Nielsen, H.B.; Karsdal, M. Bone and Soft Tissue Turnover in Relation to All-cause Mortality in Postmenopausal Women. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1098–1104. [Google Scholar] [CrossRef]
- Sambrook, P.N.; Chen, C.J.; March, L.; Cameron, I.D.; Cumming, R.G.; Lord, S.R.; Simpson, J.M.; Seibel, M.J. High bone turnover is an independent predictor of mortality in the frail elderly. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006, 21, 549–555. [Google Scholar] [CrossRef]
- Kestenbaum, B.; Katz, R.; de Boer, I.; Hoofnagle, A.; Sarnak, M.J.; Shlipak, M.G.; Jenny, N.S.; Sis-covick, D.S. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J. Am. Coll. Cardiol. 2011, 58, 1433–1441. [Google Scholar] [CrossRef] [Green Version]
- Durup, D.; Jørgensen, H.L.; Christensen, J.; Schwarz, P.; Heegaard, A.M.; Lind, B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: The CopD study. J. Clin. Endocrinol. Metab. 2012, 97, 2644–2652. [Google Scholar] [CrossRef]
- Kritchevsky, S.B.; Tooze, J.A.; Neiberg, R.H.; Schwartz, G.G.; Hausman, D.B.; Johnson, M.A.; Bauer, D.C.; Cauley, J.A.; Shea, M.K.; Cawthon, P.M.; et al. 25-Hydroxyvitamin D, parathyroid hormone, and mortality in black and white older adults: The health ABC study. J. Clin. Endocrinol. Metab. 2012, 97, 4156–4165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hilali, J.; de Koning, E.J.; van Ballegooijen, A.J.; Lips, P.; Sohl, E.; van Marwijk, H.W.J.; Visser, M.; van Schoor, N.M. Vitamin D, PTH and the risk of overall and disease-specific mortality: Results of the Longitudinal Aging Study Amsterdam. J. Steroid Biochem. Mol. Biol. 2016, 164, 386–394. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Total | Alive Throughout the Study | Dead during the Study | p Value |
|---|---|---|---|---|
| Participants (%) | 952 (100.0) | 200 (21.1) | 752 (78.9) | - |
| Follow-up time (months) Median (IQR) | 32 (15, 55) | 61 (56, 71) | 24 (12, 41) | <0.001 |
| Age (Years) Median (IQR) | 102 (100, 104) | 101 (100, 104) | 102 (100, 104) | 0.552 |
| Female (%) | 775 (81.4) | 167 (83.5) | 608 (80.9) | 0.392 |
| Hypertension (%) | 719 (75.5) | 156 (78.0) | 563 (78.9) | 0.360 |
| Diabetes mellitus (%) | 94 (9.9) | 18 (9.0) | 76 (10.1) | 0.641 |
| Previous fractures (%) | 82 (8.6) | 13 (6.5) | 69 (9.2) | 0.231 |
| Calcium (mmol/L) (Mean ± SD) (Normal range 2.15–2.55) | 2.21 ± 0.11 | 2.23 ± 0.11 | 2.21 ± 0.11 | 0.005 |
| Phosphorus (mmol/L) (Mean ± SD) (Normal range 0.89–1.6) | 1.06 ± 0.17 | 1.09 ± 0.18 | 1.05 ± 0.16 | 0.003 |
| ALP (U/L) Median (IQR) (Normal range 0–130) | 81 (67, 102) | 76 (64, 96) | 83 (67, 103) | 0.010 |
| P1NP (μg/L) Median (IQR) (Normal range 19–84) | 65.03 (47.00, 91.00) | 61.50 (44.25, 85.00) | 66.00 (48.00, 92.75) | 0.090 |
| Osteocalcin (ng/mL) Median (IQR) (Normal range 11–48) | 29.28 (20.53, 40.67) | 27.58 (19.65, 36.37) | 29.68 (20.79, 41.77) | 0.010 |
| 25(OH)D (ng/mL) Median (IQR) (Normal range 30–100) | 21.60 (16.50, 28.10) | 24.60 (19.93, 31.33) | 21.15 (15.90, 26.80) | <0.001 |
| PTH (pg/mL) Median (IQR) (Normal range 15–65) | 43.91 (31.81, 60.76) | 38.08 (29.90, 49.68) | 45.70 (32.51, 63.27) | <0.001 |
| β-CTX (ng/mL) Median (IQR) (Normal range 0.55–1.01) | 0.40 (0.25, 0.58) | 0.34 (0.23, 0.49) | 0.42 (0.26, 0.61) | <0.001 |
| Variables | Grouped by Interquartile Values | Grouped by Median Values | Grouped by Clinical Values (Three Groups) | Grouped by Clinical Values (Two Groups) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D (ng/mL) | Quantile 1 (<16.6, N = 239) | Quantile 2 (16.6–21.5, N = 225) | Quantile 3 (21.5–28.1, N = 253) | Quantile 4 (>28.1, N = 235) | <21.5 (N = 458) | ≥21.5 (N = 494) | <10 (N = 65) | 10–30 (N = 704) | ≥30 (N = 183) | <30 (N = 769) | ≥30 (N = 183) |
| Unadjusted | 1.84 (1.49, 2.26) | 1.51 (1.22, 1.86) | 1.35 (1.10, 1.67) | 1.00 (Ref) | 1.42 (1.23, 1.64) | 1.00 (Ref) | 2.05 (1.50, 2.81) | 1.44 (1.18, 1.75) | 1.00 (Ref) | 1.48 (1.22, 1.79) | 1.00 (Ref) |
| p values | <0.001 | <0.001 | 0.005 | - | <0.001 | - | <0.001 | <0.001 | - | <0.001 | - |
| Adjusted | 1.72 (1.38, 2.14) | 1.53 (1.24, 1.91) | 1.46 (1.18, 1.81) | 1.00 (Ref) | 1.32 (1.13, 1.53) | 1.00 (Ref) | 1.86 (1.34, 2.58) | 1.52 (1.25, 1.87) | 1.00 (Ref) | 1.52 (1.24, 1.86) | 1.00 (Ref) |
| p values | <0.001 | <0.001 | 0.001 | - | <0.001 | - | <0.001 | <0.001 | - | <0.001 | - |
| PTH (pg/mL) | Quantile 1 (<31.82, N = 238) | Quantile 2 (31.82–43.89, N = 238) | Quantile 3 (43.89–60.68, N = 238) | Quantile 4 (>60.68, N = 238) | ≤43.89 (N = 476) | >43.89 (N = 476) | <15 (N = 37) | 15–65 (N = 721) | >65 (N = 194) | ≤65 (N = 758) | >65 (N = 194) |
| Unadjusted | 1.02 (0.83, 1.26) | 1.00 (Ref) | 1.17 (0.95, 1.44) | 1.71 (1.40, 2.09) | 1.00 (Ref) | 1.38 (1.20, 1.60) | 1.01 (0.69, 1.46) | 1.00 (Ref) | 1.61 (1.36, 1.92) | 1.00 (Ref) | 1.61 (1.36, 1.91) |
| p values | 0.831 | - | 0.136 | <0.001 | - | <0.001 | 0.979 | - | <0.001 | - | <0.001 |
| Adjusted | 1.09 (0.88, 1.35) | 1.00 (Ref) | 1.15 (0.94, 1.42) | 1.43 (1.16, 1.76) | 1.00 (Reference) | 1.22 (1.05, 1.42) | 1.09 (0.75, 1.59) | 1.00 (Ref) | 1.30 (1.08, 1.56) | 1.00 (Ref) | 1.30 (1.08, 1.56) |
| p values | 0.417 | - | 0.182 | 0.001 | - | 0.011 | 0.643 | - | 0.005 | - | 0.005 |
| β-CTX (ng/mL) (Interquartile) | Quantile 1 (<0.248, N = 240) | Quantile 2 (0.248–0.406, N = 237) | Quantile 3 (0.406–0.579, N = 238) | Quantile 4 (>0.579, N = 237) | <0.406 (N = 477) | ≥0.406 (N = 475) | <0.55 (N = 680) | 0.55–1.01 (N = 237) | >1.01 (N = 35) | <0.55 (N = 680) | ≥0.55 (N = 272) |
| Unadjusted | 1.05 (0.85, 1.29) | 1.00 (Ref) | 1.17 (0.95, 1.44) | 1.70 (1.39, 2.08) | 1.00 (Ref) | 1.37 (1.19, 1.58) | 1.00 (Ref) | 1.36 (1.15, 1.60) | 2.30 (1.61, 3.28) | 1.00 (Ref) | 1.44 (1.23, 1.68) |
| p values | 0.670 | - | 0.129 | <0.001 | - | <0.001 | - | <0.001 | <0.001 | - | <0.001 |
| Adjusted | 1.17 (0.95, 1.45) | 1.00 (Ref) | 1.11 (0.90, 1.36) | 1.60 (1.29, 1.97) | 1.00 (Ref) | 1.21 (1.04, 1.40) | 1.00 (Ref) | 1.23 (1.04, 1.47) | 2.03 (1.41, 2.92) | 1.00 (Ref) | 1.30 (1.10, 1.54) |
| p values | 0.137 | - | 0.341 | <0.001 | - | 0.016 | - | 0.018 | <0.001 | - | 0.002 |
| Groups | Sample Size (N) | 25(OH)D (ng/mL) | PTH (pg/mL) | β-CTX (ng/mL) | Unadjusted HR, 95% CI and p Values | Adjusted HR, 95% CI and p Values | |
|---|---|---|---|---|---|---|---|
| Grouped by median values | G3 (3 risk factors) | 174 | <21.5 | >43.89 | ≥0.406 | 2.20 (1.73, 2.79) (p < 0.001) | 2.02 (1.58, 2.59) (p < 0.001) |
| G2 (2 risk factors) | 282 | <21.5 | >43.89 | <0.406 | 1.51 (1.21, 1.88) (p < 0.001) | 1.49 (1.19, 1.87) (p = 0.001) | |
| ≤43.89 | ≥0.406 | ||||||
| ≥21.5 | >43.89 | ≥0.406 | |||||
| G1 (1 risk factor) | 323 | ≥21.5 | >43.89 | <0.406 | 1.17 (0.94, 1.46) (p = 0.171) | 1.16 (0.93, 1.45) (p = 0.196) | |
| ≥21.5 | ≤43.89 | ≥0.406 | |||||
| <21.5 | ≤43.89 | <0.406 | |||||
| G0 (0 risk factor) | 173 | ≥21.5 | ≤43.89 | <0.406 | 1.00 (Reference) | 1.00 (Reference) | |
| Grouped by clinical reference values | G3 (3 risk factors) | 82 | <30 | >65 | ≥0.55 | 2.82 (2.05, 3.87) (p < 0.001) | 2.77 (1.99, 3.85) (p < 0.001) |
| G2 (2 risk factors) | 240 | <30 | >65 | <0.55 | 1.89 (1.45, 2.45) (p < 0.001) | 1.92 (1.46, 2.52) (p < 0.001) | |
| ≤65 | ≥0.55 | ||||||
| ≥30 | >65 | ≥0.55 | |||||
| G1 (1 risk factor) | 509 | ≥30 | >65 | <0.55 | 1.32 (1.03, 1.68) (p = 0.026) | 1.42 (1.11, 1.82) (p = 0.006) | |
| ≥30 | ≤65 | ≥0.55 | |||||
| <30 | ≤65 | <0.55 | |||||
| G0 (0 risk factor) | 121 | ≥30 | ≤65 | <0.55 | 1.00 (Reference) | 1.00 (Reference) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Cheng, X.; Fu, S.; Sun, D.; Zhang, W.; Liu, W.; Miao, X.; Luo, Q.; Li, H.; Zhang, J.; et al. Associations of Serum 25(OH)D, PTH, and β-CTX Levels with All-Cause Mortality in Chinese Community-Dwelling Centenarians. Nutrients 2023, 15, 94. https://doi.org/10.3390/nu15010094
Wang B, Cheng X, Fu S, Sun D, Zhang W, Liu W, Miao X, Luo Q, Li H, Zhang J, et al. Associations of Serum 25(OH)D, PTH, and β-CTX Levels with All-Cause Mortality in Chinese Community-Dwelling Centenarians. Nutrients. 2023; 15(1):94. https://doi.org/10.3390/nu15010094
Chicago/Turabian StyleWang, Bin, Xiaowei Cheng, Shihui Fu, Ding Sun, Weiguang Zhang, Weicen Liu, Xinyu Miao, Qing Luo, Hao Li, Jie Zhang, and et al. 2023. "Associations of Serum 25(OH)D, PTH, and β-CTX Levels with All-Cause Mortality in Chinese Community-Dwelling Centenarians" Nutrients 15, no. 1: 94. https://doi.org/10.3390/nu15010094