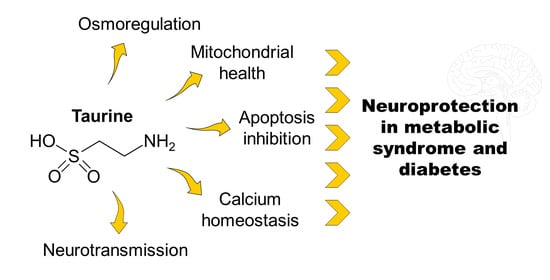
Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes
Abstract
:1. Introduction
2. Taurine Homeostasis
2.1. Brain Taurine Transport
2.2. Taurine Metabolism
2.3. Sulphur-Containing Amino Acids
3. Taurine in Cellular Physiology
3.1. Osmoregulation by Taurine
3.2. Taurine as a Neurotransmitter
3.3. Modulation of Taurine Release in the CNS
3.4. Taurine in Mitochondria
3.5. Taurine as an Inhibitor of Apoptosis
4. Brain Taurine in Diabetes
4.1. Brain Taurine Levels in Subjects with Alzheimer’s Disease
4.2. Plasma Taurine Levels in Individuals with Dementia and Alzheimer’s Disease
4.3. Brain Taurine Levels in AD Models
5. Neuroprotection by Taurine
5.1. Taurine Affords Neuroprotection in Diabetes Models
5.2. Taurine Effectiveness in Diabetes Management
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| GAT-2 | GABA transporter (SLC6A13) |
| MRS | Magnetic resonance spectroscopy |
| NMDA | N-methyl-D-aspartate |
| TauT | taurine transporter (SLC6A6) |
| VRAC | volume-regulated anion channel |
References
- Russell, D.W. The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, J. Heart Failure Research with Taurine Group Long-Term Effect of Taurine in Congestive Heart Failure: Preliminary Report. Adv. Exp. Med. Biol. 1994, 359, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Milei, J.; Ferreira, R.; Llesuy, S.F.; Forcada, P.; Covarrubias, J.; Boveris, A. Reduction of reperfusion injury with preoperative rapid intravenous infusion of taurine during myocardial revascularization. Am. Hear. J. 1992, 123, 339–345. [Google Scholar] [CrossRef]
- Zhao, H.; Qu, J.; Li, Q.; Cui, M.; Wang, J.; Zhang, K.; Liu, X.; Feng, H.; Chen, Y. Taurine supplementation reduces neuroinflammation and protects against white matter injury after intracerebral hemorrhage in rats. Amino Acids 2017, 50, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.N.; Wang, Q. Taurine and Niacin Offer a Novel Therapeutic Modality in Prevention of Chemically-Induced Pulmonary Fibrosis in Hamsters. Adv. Exp. Med. Biol. 1992, 315, 329–340. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Brandao, C.F.C.; Muñoz, V.R.; Batitucci, G.; Tavares, M.E.D.A.; Teixeira, G.R.; Pauli, J.R.; De Moura, L.P.; Ropelle, E.R.; Cintra, D.E.; et al. Taurine supplementation in conjunction with exercise modulated cytokines and improved subcutaneous white adipose tissue plasticity in obese women. Amino Acids 2021, 53, 1391–1403. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.-H.; Uddin, M.S.; Kim, I.-S.; Choi, D.-K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Yeon, J.-A.; Kim, S.-J. Neuroprotective Effect of Taurine against Oxidative Stress-Induced Damages in Neuronal Cells. Biomol. Ther. 2010, 18, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Rezaee-Tazangi, F.; Zeidooni, L.; Rafiee, Z.; Fakhredini, F.; Kalantari, H.; Alidadi, H.; Khorsandi, L. Taurine effects on Bisphenol A-induced oxidative stress in the mouse testicular mitochondria and sperm motility. JBRA Assist. Reprod. 2020, 24, 428–435. [Google Scholar] [CrossRef]
- Nakajima, Y.; Osuka, K.; Seki, Y.; Gupta, R.C.; Hara, M.; Takayasu, M.; Wakabayashi, T. Taurine Reduces Inflammatory Responses after Spinal Cord Injury. J. Neurotrauma 2010, 27, 403–410. [Google Scholar] [CrossRef]
- Albrecht, J.; Schousboe, A. Taurine Interaction with Neurotransmitter Receptors in the CNS: An Update. Neurochem. Res. 2005, 30, 1615–1621. [Google Scholar] [CrossRef]
- Oja, S.S.; Saransaari, P. Significance of Taurine in the Brain. Adv. Exp. Med. Biol. 2017, 1, 89–94. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [Green Version]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Wharton, B.; Morley, R.; Isaacs, E.B.; Cole, T.J.; Lucas, A. Low plasma taurine and later neurodevelopment. Arch. Dis. Child.-Fetal Neonatal Ed. 2004, 89, F497–F498. [Google Scholar] [CrossRef] [Green Version]
- Sturman, J.; Moretz, R.; French, J.; Wisniewski, H. Taurine deficiency in the developing cat: Persistence of the cerebellar external granule cell layer. J. Neurosci. Res. 1985, 13, 405–416. [Google Scholar] [CrossRef]
- Rak, K.; Völker, J.; Jürgens, L.; Scherzad, A.; Schendzielorz, P.; Radeloff, A.; Jablonka, S.; Mlynski, R.; Hagen, R. Neurotrophic effects of taurine on spiral ganglion neurons in vitro. NeuroReport 2014, 25, 1250–1254. [Google Scholar] [CrossRef]
- Mersman, B.; Zaidi, W.; Syed, N.I.; Xu, F. Taurine Promotes Neurite Outgrowth and Synapse Development of Both Vertebrate and Invertebrate Central Neurons. Front. Synaptic Neurosci. 2020, 12, 29. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, L.; Xiao, Q.; Wang, Y.; Meng, X.; Xu, R.; Wang, S.; Na, R. Obesity and diabetes related plasma amino acid alterations. Clin. Biochem. 2013, 46, 1447–1452. [Google Scholar] [CrossRef]
- De Luca, G.; Calpona, P.; Caponetti, A.; Romano, G.; Di Benedetto, A.; Cucinotta, D.; Di Giorgio, R. Taurine and osmoregulation: Platelet taurine content, uptake, and release in type 2 diabetic patients. Metabolism 2001, 50, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Graham, I.M.; Reis, R.P.; Luis, A.S.; Smith, A.D.; Refsum, H. The association of fasting plasma sulfur-containing compounds with BMI, serum lipids and apolipoproteins. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 1031–1038. [Google Scholar] [CrossRef]
- Tessari, P.; Kiwanuka, E.; Coracina, A.; Zaramella, M.; Vettore, M.; Valerio, A.; Garibotto, G. Insulin in methionine and homocysteine kinetics in healthy humans: Plasma vs. intracellular models. Am. J. Physiol. Metab. 2005, 288, E1270–E1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berson, E.L.; Schmidt, S.Y.; Rabin, A.R. Plasma amino-acids in hereditary retinal disease. Ornithine, lysine, and taurine. Br. J. Ophthalmol. 1976, 60, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarla, C.; Giovannini, I.; Siegel, J.H.; Boldrini, G.; Castagneto, M. The Relationship between Plasma Taurine and Other Amino Acid Levels in Human Sepsis. J. Nutr. 2000, 130, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.M.; Mühling, J.; Weiss, S.; Kärcher, B.; Lohr, T.; Menges, T.; Little, S.; Hempelmann, G. Relationship of taurine and other amino acids in plasma and in neutrophils of septic trauma patients. Amino Acids 2005, 30, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.K.; Sanders, T.A.B. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br. J. Nutr. 1986, 56, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, M.-S.; Rodrigo, G.C.; Chapman, R. Interdependence of intracellular taurine and sodium in guinea pig heart. Cardiovasc. Res. 1992, 26, 897–905. [Google Scholar] [CrossRef]
- Schønheyder, F.; Lyngbye, J. Influence of partial starvation and of acute scurvy on the free amino acids in blood plasma and muscle in the guinea-pig. Br. J. Nutr. 1962, 16, 75–82. [Google Scholar] [CrossRef]
- Lerma, J.; Herranz, A.; Herreras, O.; Abraira, V.; del Rio, R.M. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986, 384, 145–155. [Google Scholar] [CrossRef]
- Trachtman, H.; Futterweit, S.; Sturman, J.A. Cerebral Taurine Transport Is Increased During Streptozocin-Induced Diabetes in Rats. Diabetes 1992, 41, 1130–1140. [Google Scholar] [CrossRef]
- Brand, H.S.; Chamuleau, R.A.F.M.; Jörning, G.G. Changes in urinary taurine and hypotaurine excretion after two-thirds hepatectomy in the rat. Amino Acids 1998, 15, 373–383. [Google Scholar] [CrossRef]
- Larsen, L.H.; Ørstrup, L.K.H.; Hansen, S.H.; Grunnet, N.; Quistorff, B.; Mortensen, O.H. Fructose Feeding Changes Taurine Homeostasis in Wistar Rats. Adv. Exp. Med. Biol. 2015, 803, 695–706. [Google Scholar] [CrossRef]
- Cardoso, S.; Carvalho, C.; Santos, R.; Correia, S.; Santos, M.S.; Seiça, R.; Oliveira, C.R.; Moreira, P.I. Impact of STZ-induced hyperglycemia and insulin-induced hypoglycemia in plasma amino acids and cortical synaptosomal neurotransmitters. Synapse 2010, 65, 457–466. [Google Scholar] [CrossRef]
- Ma, Y.; Maruta, H.; Sun, B.; Wang, C.; Isono, C.; Yamashita, H. Effects of long-term taurine supplementation on age-related changes in skeletal muscle function of Sprague–Dawley rats. Amino Acids 2021, 53, 159–170. [Google Scholar] [CrossRef]
- Chesney, R.W.; Jax, D.K. Developmental Aspects of Renal beta-Amino Acid Transport, I. Ontogeny of Taurine Reabsorption and Accumulation in Rat Renal Cortex. Pediatr. Res. 1979, 13, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Warskulat, U.; Borsch, E.; Reinehr, R.; Heller-Stilb, B.; Mönnighoff, I.; Buchczyk, D.; Donner, M.; Flögel, U.; Kappert, G.; Soboll, S.; et al. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006, 20, 574–576. [Google Scholar] [CrossRef]
- Garcia-Serrano, A.M.; Mohr, A.A.; Philippe, J.; Skoug, C.; Spégel, P.; Duarte, J.M.N. Cognitive Impairment and Metabolite Profile Alterations in the Hippocampus and Cortex of Male and Female Mice Exposed to a Fat and Sugar-Rich Diet are Normalized by Diet Reversal. Aging Dis. 2022, 13, 267. [Google Scholar] [CrossRef]
- Tao, Y.; He, M.; Yang, Q.; Ma, Z.; Qu, Y.; Chen, W.; Peng, G.; Teng, D. Systemic taurine treatment provides neuroprotection against retinal photoreceptor degeneration and visual function impairments. Drug Des. Dev. Ther. 2019, 13, 2689–2702. [Google Scholar] [CrossRef] [Green Version]
- Taranukhin, A.G.; Taranukhina, E.Y.; Saransaari, P.; Podkletnova, I.M.; Pelto-Huikko, M.; Oja, S.S. Neuroprotection by taurine in ethanol-induced apoptosis in the developing cerebellum. J. Biomed. Sci. 2010, 17, S12. [Google Scholar] [CrossRef] [Green Version]
- Hadj-Saïd, W.; Froger, N.; Ivkovic, I.; Jiménez-López, M.; Dubus, É.; Dégardin-Chicaud, J.; Simonutti, M.; Quénol, C.; Neveux, N.; Villegas-Pérez, M.P.; et al. Quantitative and Topographical Analysis of the Losses of Cone Photoreceptors and Retinal Ganglion Cells Under Taurine Depletion. Investig. Opthalmol. Vis. Sci. 2016, 57, 4692–4703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambarota, G.; Mekle, R.; Xin, L.; Hergt, M.; Van Der Zwaag, W.; Krueger, G.; Gruetter, R. In vivo measurement of glycine with short echo-time 1H MRS in human brain at 7 T. Magn. Reson. Mater. Phys. Biol. Med. 2008, 22, 1–4. [Google Scholar] [CrossRef]
- Mekle, R.; Mlynarik, V.; Gambarota, G.; Hergt, M.; Krueger, G.; Gruetter, R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn. Reson. Med. 2009, 61, 1279–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deelchand, D.K.; Van de Moortele, P.-F.; Adriany, G.; Iltis, I.; Andersen, P.; Strupp, J.P.; Vaughan, J.T.; Uğurbil, K.; Henry, P.-G. In vivo1H NMR spectroscopy of the human brain at 9.4T: Initial results. J. Magn. Reson. 2010, 206, 74–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, B.; Mekle, R.; Xin, L.; Kunz, N.; Gruetter, R. Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 tesla. J. Neurosci. Res. 2013, 91, 1076–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marjańska, M.; Auerbach, E.J.; Valabrègue, R.; Van de Moortele, P.-F.; Adriany, G.; Garwood, M. Localized1H NMR spectroscopy in different regions of human brainin vivoat 7 T:T2relaxation times and concentrations of cerebral metabolites. NMR Biomed. 2011, 25, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Marjańska, M.; McCarten, J.R.; Hodges, J.; Hemmy, L.S.; Grant, A.; Deelchand, D.K.; Terpstra, M. Region-specific aging of the human brain as evidenced by neurochemical profiles measured noninvasively in the posterior cingulate cortex and the occipital lobe using 1 H magnetic resonance spectroscopy at 7 T. Neuroscience 2017, 354, 168–177. [Google Scholar] [CrossRef]
- Ueki, I.; Roman, H.B.; Valli, A.; Fieselmann, K.; Lam, J.; Peters, R.; Hirschberger, L.L.; Stipanuk, M.H. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am. J. Physiol. Metab. 2011, 301, E668–E684. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-T.; Lee, P.; Dong, Y.; Yeh, H.-W.; Kim, J.; Weiner, C.P.; Brooks, W.M.; Choi, I.-Y. In Vivo Neurochemical Characterization of Developing Guinea Pigs and the Effect of Chronic Fetal Hypoxia. Neurochem. Res. 2016, 41, 1831–1843. [Google Scholar] [CrossRef]
- Lei, H.; Berthet, C.; Hirt, L.; Gruetter, R. Evolution of the Neurochemical Profile after Transient Focal Cerebral Ischemia in the Mouse Brain. J. Cereb. Blood Flow Metab. 2009, 29, 811–819. [Google Scholar] [CrossRef]
- Lei, H.; Duarte, J.M.; Mlynarik, V.; Python, A.; Gruetter, R. Deep thiopental anesthesia alters steady-state glucose homeostasis but not the neurochemical profile of rat cortex. J. Neurosci. Res. 2009, 88, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Gambarota, G.; Duarte, J.M.N.; Mlynárik, V.; Gruetter, R. Direct in vivo measurement of glycine and the neurochemical profile in the rat medulla oblongata. NMR Biomed. 2010, 23, 1097–1102. [Google Scholar] [CrossRef]
- Harris, J.L.; Yeh, H.-W.; Swerdlow, R.H.; Choi, I.-Y.; Lee, P.; Brooks, W.M. High-field proton magnetic resonance spectroscopy reveals metabolic effects of normal brain aging. Neurobiol. Aging 2014, 35, 1686–1694. [Google Scholar] [CrossRef] [Green Version]
- Sonnay, S.; Duarte, J.M.; Just, N.; Gruetter, R. Compartmentalised energy metabolism supporting glutamatergic neurotransmission in response to increased activity in the rat cerebral cortex: A 13C MRS study in vivo at 14.1 T. J. Cereb. Blood Flow Metab. 2016, 36, 928–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuellar-Baena, S.; Landeck, N.; Sonnay, S.; Buck, K.; Mlynarik, V.; Zandt, R.I.; Kirik, D. Assessment of brain metabolite correlates of adeno-associated virus-mediated over-expression of human alpha-synuclein in cortical neurons by in vivo 1 H-MR spectroscopy at 9.4 T. J. Neurochem. 2016, 137, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Kulak, A.; Duarte, J.M.N.; Do, K.Q.; Gruetter, R. Neurochemical profile of the developing mouse cortex determined by in vivo1H NMR spectroscopy at 14.1 T and the effect of recurrent anaesthesia. J. Neurochem. 2010, 115, 1466–1477. [Google Scholar] [CrossRef] [Green Version]
- Das Neves Duarte, J.M.; Kulak, A.; Gholam-Razaee, M.M.; Cuenod, M.; Gruetter, R.; Do, K.Q. N-Acetylcysteine Normalizes Neurochemical Changes in the Glutathione-Deficient Schizophrenia Mouse Model During Development. Biol. Psychiatry 2012, 71, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, J.M.; Do, K.Q.; Gruetter, R. Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiol. Aging 2014, 35, 1660–1668. [Google Scholar] [CrossRef]
- Corcoba, A.; Steullet, P.; Duarte, J.; van de Looij, Y.; Monin, A.; Cuenod, M.; Gruetter, R.; Do, K.Q. Glutathione Deficit Affects the Integrity and Function of the Fimbria/Fornix and Anterior Commissure in Mice: Relevance for Schizophrenia. Int. J. Neuropsychopharmacol. 2016, 19, pyv110. [Google Scholar] [CrossRef] [Green Version]
- Gapp, K.; Corcoba, A.; Van Steenwyk, G.; Mansuy, I.M.; Duarte, J.M. Brain metabolic alterations in mice subjected to postnatal traumatic stress and in their offspring. J. Cereb. Blood Flow Metab. 2016, 37, 2423–2432. [Google Scholar] [CrossRef]
- Roig-Pérez, S.; Moretó, M.; Ferrer, R. Transepithelial Taurine Transport in Caco-2 Cell Monolayers. J. Membr. Biol. 2005, 204, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.G.; Smith, L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–511. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W.; Lippincott, S.; Gusowski, N.; Padilla, M.; Zelikovic, I. Studies on Renal Adaptation to Altered Dietary Amino Acid Intake: Tissue Taurine Responses in Nursing and Adult Rats. J. Nutr. 1986, 116, 1965–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaeomor, A.; Wyss, J.M.; Jirakulsomchok, D.; Roysommuti, S. High sugar intake via the renin-angiotensin system blunts the baroreceptor reflex in adult rats that were perinatally depleted of taurine. J. Biomed. Sci. 2010, 17, S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik, O.P.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Costa, M.; Chen, Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010, 208, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Rasgado-Flores, H.; Mokashi, A.; Hawkins, R.A. Na+-dependent transport of taurine is found only on the abluminal membrane of the blood–brain barrier. Exp. Neurol. 2012, 233, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Senmaru, M.; Terasaki, T.; Tsuji, A. Na+- and Cl−-Dependent transport of taurine at the blood-brain barrier. Biochem. Pharmacol. 1995, 50, 1783–1793. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Kang, Y.-S. The brain-to-blood efflux transport of taurine and changes in the blood–brain barrier transport system by tumor necrosis factor-α. Brain Res. 2004, 1023, 141–147. [Google Scholar] [CrossRef]
- Zhou, Y.; Holmseth, S.; Guo, C.; Hassel, B.; Höfner, G.; Huitfeldt, H.S.; Wanner, K.; Danbolt, N.C. Deletion of the γ-Aminobutyric Acid Transporter 2 (GAT2 and SLC6A13) Gene in Mice Leads to Changes in Liver and Brain Taurine Contents. J. Biol. Chem. 2012, 287, 35733–35746. [Google Scholar] [CrossRef] [Green Version]
- Geier, E.G.; Chen, E.C.; Webb, A.; Papp, A.C.; Yee, S.W.; Sadee, W.; Giacomini, K.M. Profiling Solute Carrier Transporters in the Human Blood–Brain Barrier. Clin. Pharmacol. Ther. 2013, 94, 636–639. [Google Scholar] [CrossRef]
- Nishimura, T.; Higuchi, K.; Yoshida, Y.; Sugita-Fujisawa, Y.; Kojima, K.; Sugimoto, M.; Santo, M.; Tomi, M.; Nakashima, E. Hypotaurine Is a Substrate of GABA Transporter Family Members GAT2/Slc6a13 and TAUT/Slc6a. Biol. Pharm. Bull. 2018, 41, 1523–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pow, D.V.; Sullivan, R.; Reye, P.; Hermanussen, S. Localization of taurine transporters, taurine, and3H taurine accumulation in the rat retina, pituitary, and brain. Glia 2002, 37, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Shimada, A.; Wada, M.; Miyakawa, S.; Yamamoto, A. Functional Expression of Taurine Transporter and its Up-Regulation in Developing Neurons from Mouse Cerebral Cortex. Pharm. Res. 2006, 23, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.M.; Smith, K.E.; Borden, L.A.; Weinshank, R.L.; Branchek, T.A.; Gustafson, E.L. Localization of messenger RNAs encoding three GABA transporters in rat brain: An in situ hybridization study. Mol. Brain Res. 1995, 33, 7–21. [Google Scholar] [CrossRef]
- Mongin, A.A. Volume-regulated anion channel—A frenemy within the brain. Pflügers Arch. Eur. J. Physiol. 2016, 468, 421–441. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, T.; Yamada, J.; Akita, T.; Matsushima, Y.; Yanagawa, Y.; Fukuda, A. Roles of taurine-mediated tonic GABAA receptor activation in the radial migration of neurons in the fetal mouse cerebral cortex. Front. Cell. Neurosci. 2014, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Brand, A.; Leibfritz, D.; Hamprecht, B.; Dringen, R. Metabolism of Cysteine in Astroglial Cells: Synthesis of Hypotaurine and Taurine. J. Neurochem. 2002, 71, 827–832. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Garg, S.K.; Banerjee, R. Taurine Biosynthesis by Neurons and Astrocytes. J. Biol. Chem. 2011, 286, 32002–32010. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Park, S.Y.; Dobkin, C.; Schuller-Levis, G. Development of a Novel Cysteine Sulfinic Acid Decarboxylase Knockout Mouse: Dietary Taurine Reduces Neonatal Mortality. J. Amino Acids 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeravalli, S.; Phillips, I.R.; Freire, R.T.; Varshavi, D.; Everett, J.R.; Shephard, E.A. Flavin-Containing Monooxygenase 1 Catalyzes the Production of Taurine from Hypotaurine. Drug Metab. Dispos. 2020, 48, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Janmohamed, A.; Hernandez, D.; Phillips, I.R.; Shephard, E. Cell-, tissue-, sex- and developmental stage-specific expression of mouse flavin-containing monooxygenases (Fmos). Biochem. Pharmacol. 2004, 68, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Junyent, F.; De Lemos, L.; Utrera, J.; Paco, S.; Aguado, F.; Camins, A.; Pallàs, M.; Romero, R.; Auladell, C. Content and traffic of taurine in hippocampal reactive astrocytes. Hippocampus 2011, 21, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Vitvitsky, V.; Garg, S.K. The undertow of sulfur metabolism on glutamatergic neurotransmission. Trends Biochem. Sci. 2008, 33, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. The Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.; Wu, Q.; Li, J.; Sun, S.; Sun, S. S-adenosylmethionine: A metabolite critical to the regulation of autophagy. Cell Prolif. 2020, 53, e12891. [Google Scholar] [CrossRef] [PubMed]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic Systems in Stress: Structural and Molecular Genetic Approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef] [PubMed]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchwell, K.B.; Wright, S.H.; Emma, F.; Rosenberg, P.; Strange, K. NMDA Receptor Activation Inhibits Neuronal Volume Regulation after Swelling Induced by Veratridine-Stimulated Na+Influx in Rat Cortical Cultures. J. Neurosci. 1996, 16, 7447–7457. [Google Scholar] [CrossRef] [Green Version]
- Murphy, T.R.; Davila, D.; Cuvelier, N.; Young, L.R.; Lauderdale, K.; Binder, D.K.; Fiacco, T.A. Hippocampal and Cortical Pyramidal Neurons Swell in Parallel with Astrocytes during Acute Hypoosmolar Stress. Front. Cell. Neurosci. 2017, 11, 275. [Google Scholar] [CrossRef] [Green Version]
- Lambert, I.H. Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem. Res. 2004, 29, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Walz, W.; Allen, A.F. Evaluation of the osmoregulatory function of taurine in brain cells. Exp. Brain Res. 1987, 68, 290–298. [Google Scholar] [CrossRef]
- Oja, S. Chloride ions, potassium stimulation and release of endogenous taurine from cerebral cortical slices from 3 day old and 3 month old mice. Neurochem. Int. 1995, 27, 313–318. [Google Scholar] [CrossRef]
- Verbalis, J.; Gullans, S. Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res. 1991, 567, 274–282. [Google Scholar] [CrossRef]
- Lien, Y.H.; Shapiro, J.; Chan, L. Effects of hypernatremia on organic brain osmoles. J. Clin. Investig. 1990, 85, 1427–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, J.E.; Goldfinger, M.D. Amino acid content of rat cerebral astrocytes adapted to hyperosmotic medium in vitro. J. Neurosci. Res. 1990, 27, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Olea, R.; Morán, J.; Pasantes-Morales, H. Changes in taurine transport evoked by hyperosmolarity in cultured astrocytes. J. Neurosci. Res. 1992, 32, 86–92. [Google Scholar] [CrossRef]
- Bitoun, M.; Tappaz, M. Taurine Down-Regulates Basal and Osmolarity-Induced Gene Expression of Its Transporter, but Not the Gene Expression of Its Biosynthetic Enzymes, in Astrocyte Primary Cultures. J. Neurochem. 2002, 75, 919–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimelberg, H.; Goderie, S.; Higman, S.; Pang, S.; Waniewski, R. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 1990, 10, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a Plasma Membrane Protein, Is an Essential Component of Volume-Regulated Anion Channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Schmid, R.; Sieghart, W.; Karobath, M. Taurine Uptake in Synaptosomal Fractions of Rat Cerebral Cortex. J. Neurochem. 1975, 25, 5–9. [Google Scholar] [CrossRef]
- Lähdesmäki, P.; Pasula, M.; Oja, S.S. Effect of electrical stimulation and chlorpromazine on the uptake and release of taurine, γ-aminobutyric acid and glutamic acid in mouse brain synaptosomes. J. Neurochem. 1975, 25, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Kontro, P.; Oja, S.S. Sodium-independent taurine binding to brain synaptic membranes. Cell. Mol. Neurobiol. 1983, 3, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.; Peterson, A. Sodium-dependent and sodium-independent binding of taurine to rat brain synaptosomes. Neurochem. Int. 1989, 14, 79–84. [Google Scholar] [CrossRef]
- Lynch, J.W. Molecular Structure and Function of the Glycine Receptor Chloride Channel. Physiol. Rev. 2004, 84, 1051–1095. [Google Scholar] [CrossRef]
- Shibanoki, S.; Kogure, M.; Sugahara, M.; Ishikawa, K. Effect of Systemic Administration of N-Methyl-d-Aspartic Acid on Extracellular Taurine Level Measured by Microdialysis in the Hippocampal CA1 Field and Striatum of Rats. J. Neurochem. 1993, 61, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Segovia, G.; Del Arco, A.; Mora, F. Endogenous Glutamate Increases Extracellular Concentrations of Dopamine, GABA, and Taurine Through NMDA and AMPA/Kainate Receptors in Striatum of the Freely Moving Rat: A Microdialysis Study. J. Neurochem. 1997, 69, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, I.; Kontro, P.; Oja, S.S. Release of preloaded taurine and hypotaurine from astrocytes in primary culture: Stimulation by calcium-free media. Neurochem. Res. 1985, 10, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Shain, W.G.; Martin, D.L. Activation of beta-adrenergic receptors stimulates taurine release from glial cells. Cell. Mol. Neurobiol. 1984, 4, 191–196. [Google Scholar] [CrossRef]
- Philibert, R.A.; Rogers, K.L.; Allen, A.J.; Dutton, G.R. Dose-Dependent, K+-Stimulated Efflux of Endogenous Taurine from Primary Astrocyte Cultures Is Ca2+-Dependent. J. Neurochem. 1988, 51, 122–126. [Google Scholar] [CrossRef]
- Philibert, R.; Rogers, K.L.; Dutton, G.R. K+-evoked taurine efflux from cerebellar astrocytes: On the roles of Ca2+ and Na+. Neurochem. Research 1989, 14, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Barakat, L.; Wang, D.; Bordey, A. Carrier-mediated uptake and release of taurine from Bergmann glia in rat cerebellar slices. J. Physiol. 2002, 541, 753–767. [Google Scholar] [CrossRef]
- Choe, K.; Olson, J.E.; Bourque, C.W. Taurine Release by Astrocytes Modulates Osmosensitive Glycine Receptor Tone and Excitability in the Adult Supraoptic Nucleus. J. Neurosci. 2012, 32, 12518–12527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCool, B.; Botting, S.K. Characterization of strychnine-sensitive glycine receptors in acutely isolated adult rat basolateral amygdala neurons. Brain Res. 2000, 859, 341–351. [Google Scholar] [CrossRef]
- Hussy, N.; Brès, V.; Rochette, M.; Duvoid, A.; Alonso, G.; Dayanithi, G.; Moos, F.C. Osmoregulation of Vasopressin Secretion via Activation of Neurohypophysial Nerve Terminals Glycine Receptors by Glial Taurine. J. Neurosci. 2001, 21, 7110–7116. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-Y.; Xu, T.-L. Taurine-evoked chloride current and its potentiation by intracellular Ca2+ in immature rat hippocampal CA1 neurons. Amino Acids 2003, 24, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Krnjević, K.; Wang, F.; Ye, J.H. Taurine Activates Strychnine-Sensitive Glycine Receptors in Neurons Freshly Isolated from Nucleus Accumbens of Young Rats. J. Neurophysiol. 2004, 91, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhou, K.-Q.; Huang, Y.-N.; Chen, L.; Xu, T.-L. Taurine activates strychnine-sensitive glycine receptors in neurons of the rat inferior colliculus. Brain Res. 2004, 1021, 232–240. [Google Scholar] [CrossRef]
- Jiang, Z.; Yue, M.; Chandra, D.; Keramidas, A.; Goldstein, P.; Homanics, G.; Harrison, N.L. Taurine Is a Potent Activator of Extrasynaptic GABAA Receptors in the Thalamus. J. Neurosci. 2008, 28, 106–115. [Google Scholar] [CrossRef] [Green Version]
- El Idrissi, A.; Trenkner, E. Growth Factors and Taurine Protect against Excitotoxicity by Stabilizing Calcium Homeostasis and Energy Metabolism. J. Neurosci. 1999, 19, 9459–9468. [Google Scholar] [CrossRef]
- Louzada, P.R.; Lima, A.C.P.; Mendonca-Silva, D.L.; Noël, F.; De Mello, F.G.; Ferreira, S.T. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: Activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 2004, 18, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Bulley, S.; Shen, W. Reciprocal regulation between taurine and glutamate response via Ca2+- dependent pathways in retinal third-order neurons. J. Biomed. Sci. 2010, 17, S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Idrissi, A.; Trenkner, E. Taurine as a Modulator of Excitatory and Inhibitory Neurotransmission. Neurochem. Res. 2004, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Saransaari, P.; Oja, S. Excitatory amino acids evoke taurine release from cerebral cortex slices from adult and developing mice. Neuroscience 1991, 45, 451–459. [Google Scholar] [CrossRef]
- Saransaari, P. Taurine release from the developing and ageing hippocampus: Stimulation by agonists of ionotropic glutamate receptors. Mech. Ageing Dev. 1997, 99, 219–232. [Google Scholar] [CrossRef]
- Saransaari, P.; Oja, S. Modulation of the ischemia-induced taurine release by adenosine receptors in the developing and adult mouse hippocampus. Neuroscience 2000, 97, 425–430. [Google Scholar] [CrossRef]
- Saransaari, P.P.; Oja, S.S. Involvement of metabotropic glutamate receptors in taurine release in the adult and developing mouse hippocampus. Amino Acids 1999, 16, 165–179. [Google Scholar] [CrossRef]
- Li, G.; Olson, J.E. Purinergic activation of anion conductance and osmolyte efflux in cultured rat hippocampal neurons. Am. J. Physiol. Physiol. 2008, 295, C1550–C1560. [Google Scholar] [CrossRef] [Green Version]
- Bonhaus, D.W.; Lippincott, S.E.; Huxtable, R.J.; Sanchez, A.P.; Scheffner, D. Subcellular Distribution of Neuroactive Amino Acids in Brains of Genetically Epileptic Rats. Epilepsia 1984, 25, 564–568. [Google Scholar] [CrossRef]
- Chen, W.; Freinkman, E.; Wang, T.; Birsoy, K.; Sabatini, D.M. Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism. Cell 2016, 166, 1324–1337.e11. [Google Scholar] [CrossRef]
- Hansen, S.H.; Andersen, M.L.; Cornett, C.; Gradinaru, R.; Grunnet, N. A role for taurine in mitochondrial function. J. Biomed. Sci. 2010, 17, S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnay, S.; Poirot, J.; Just, N.; Clerc, A.-C.; Gruetter, R.; Rainer, G.; Duarte, J.M.N. Astrocytic and neuronal oxidative metabolism are coupled to the rate of glutamate-glutamine cycle in the tree shrew visual cortex. Glia 2017, 66, 477–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Abad, M.F.; Di Benedetto, G.; Magalhães, P.J.; Filippin, L.; Pozzan, T. Mitochondrial pH Monitored by a New Engineered Green Fluorescent Protein Mutant. J. Biol. Chem. 2004, 279, 11521–11529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarias, G.; Perreten, H.; Lengacher, S.; Poburko, D.; Demaurex, N.; Magistretti, P.J.; Chatton, J.-Y. Glutamate Transport Decreases Mitochondrial pH and Modulates Oxidative Metabolism in Astrocytes. J. Neurosci. 2011, 31, 3550–3559. [Google Scholar] [CrossRef]
- Poburko, D.; Domingo, J.S.; Demaurex, N. Dynamic Regulation of the Mitochondrial Proton Gradient during Cytosolic Calcium Elevations. J. Biol. Chem. 2011, 286, 11672–11684. [Google Scholar] [CrossRef] [Green Version]
- Thevenet, J.; De Marchi, U.; Domingo, J.S.; Christinat, N.; Bultot, L.; Lefebvre, G.; Sakamoto, K.; Descombes, P.; Masoodi, M.; Wiederkehr, A. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J. 2016, 30, 1913–1926. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, H.; Ommati, M.M.; Farshad, O.; Jamshidzadeh, A.; Nikbakht, M.R.; Niknahad, H.; Heidari, R. Taurine and isolated mitochondria: A concentration-response study. Trends Pharm. Sci. 2019, 5, 197–206. [Google Scholar]
- Niknahad, H.; Jamshidzadeh, A.; Heidari, R.; Zarei, M.; Ommati, M.M. Ammonia-induced mitochondrial dysfunction and energy metabolism disturbances in isolated brain and liver mitochondria, and the effect of taurine administration: Relevance to hepatic encephalopathy treatment. Clin. Exp. Hepatol. 2017, 3, 141–151. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem. J. 1988, 256, 251–255. [Google Scholar] [CrossRef]
- Parvez, S.; Tabassum, H.; Banerjee, B.D.; Raisuddin, S. Taurine Prevents Tamoxifen-Induced Mitochondrial Oxidative Damage in Mice. Basic Clin. Pharmacol. Toxicol. 2008, 102, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Wada, T.; Saigo, K.; Watanabe, K. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002, 21, 6581–6589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasukawa, T.; Kirino, Y.; Ishii, N.; Holt, I.; Jacobs, H.T.; Makifuchi, T.; Fukuhara, N.; Ohta, S.; Suzuki, T.; Watanabe, K. Wobble modification deficiency in mutant tRNAs in patients with mitochondrial diseases. FEBS Lett. 2005, 579, 2948–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakruddin; Wei, F.-Y.; Suzuki, T.; Asano, K.; Kaieda, T.; Omori, A.; Izumi, R.; Fujimura, A.; Kaitsuka, T.; Miyata, K.; et al. Defective Mitochondrial tRNA Taurine Modification Activates Global Proteostress and Leads to Mitochondrial Disease. Cell Rep. 2018, 22, 482–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, S.W.; Jong, C.J.; Ito, T.; Azuma, J. Role of taurine in the pathologies of MELAS and MERRF. Amino Acids 2012, 46, 47–56. [Google Scholar] [CrossRef]
- Ohsawa, Y.; Hagiwara, H.; Nishimatsu, S.-I.; Hirakawa, A.; Kamimura, N.; Ohtsubo, H.; Fukai, Y.; Murakami, T.; Koga, Y.; Goto, Y.-I.; et al. Taurine supplementation for prevention of stroke-like episodes in MELAS: A multicentre, open-label, 52-week phase III trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 529–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Li, H.; Hou, D.; Hou, J. Taurine Pretreatment Prevents Isoflurane-Induced Cognitive Impairment by Inhibiting ER Stress-Mediated Activation of Apoptosis Pathways in the Hippocampus in Aged Rats. Neurochem. Res. 2016, 41, 2517–2525. [Google Scholar] [CrossRef]
- Li, S.; Yang, L.; Zhang, Y.; Zhang, C.; Shao, J.; Liu, X.; Li, Y.; Piao, F. Taurine Ameliorates Arsenic-Induced Apoptosis in the Hippocampus of Mice Through Intrinsic Pathway. Adv. Exp. Med. Biol. 2017, 975, 183–192. [Google Scholar] [CrossRef]
- Agarwal, R.; Arfuzir, N.N.N.; Iezhitsa, I.; Agarwal, P.; Sidek, S.; Ismail, N.M. Taurine protects against retinal and optic nerve damage induced by endothelin-1 in rats via antioxidant effects. Neural Regen. Res. 2018, 13, 2014. [Google Scholar] [CrossRef]
- Sun, M.; Xu, C. Neuroprotective Mechanism of Taurine due to Up-regulating Calpastatin and Down-regulating Calpain and Caspase-3 during Focal Cerebral Ischemia. Cell. Mol. Neurobiol. 2007, 28, 593–611. [Google Scholar] [CrossRef]
- Sun, M.; Gu, Y.; Zhao, Y.; Xu, C. Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 2011, 40, 1419–1429. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Ma, P.-S.; Wu, W.; Zhou, R.; Hao, Y.-J.; Niu, Y.; Sun, T.; Li, Y.-X.; Yu, J.-Q. Neuroprotective actions of taurine on hypoxic-ischemic brain damage in neonatal rats. Brain Res. Bull. 2016, 124, 295–305. [Google Scholar] [CrossRef]
- Gharibani, P.M.; Modi, J.; Pan, C.; Menzie, J.; Ma, Z.; Chen, P.-C.; Tao, R.; Prentice, H.; Wu, J.-Y. The Mechanism of Taurine Protection Against Endoplasmic Reticulum Stress in an Animal Stroke Model of Cerebral Artery Occlusion and Stroke-Related Conditions in Primary Neuronal Cell Culture. Adv. Exp. Med. Biol. 2013, 776, 241–258. [Google Scholar] [CrossRef]
- Gharibani, P.; Modi, J.; Menzie, J.; Alexandrescu, A.; Ma, Z.; Tao, R.; Prentice, H.; Wu, J.-Y. Comparison between single and combined post-treatment with S-Methyl-N,N-diethylthiolcarbamate sulfoxide and taurine following transient focal cerebral ischemia in rat brain. Neuroscience 2015, 300, 460–473. [Google Scholar] [CrossRef]
- Duarte, J.M.N. Metabolic Alterations Associated to Brain Dysfunction in Diabetes. Aging Dis. 2014, 6, 304–321. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Serrano, A.M.; Duarte, J.M.N. Brain Metabolism Alterations in Type 2 Diabetes: What Did We Learn from Diet-Induced Diabetes Models? Front. Neurosci. 2020, 14, 229. [Google Scholar] [CrossRef] [Green Version]
- Duarte, J.M.N.; Carvalho, R.; Cunha, R.; Gruetter, R. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. J. Neurochem. 2009, 111, 368–379. [Google Scholar] [CrossRef]
- Wang, W.-T.; Lee, P.; Yeh, H.-W.; Smirnova, I.V.; Choi, I.-Y. Effects of acute and chronic hyperglycemia on the neurochemical profiles in the rat brain with streptozotocin-induced diabetes detected using in vivo1H MR spectroscopy at 9.4 T. J. Neurochem. 2012, 121, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Duarte, J.M.N.; Skoug, C.; Silva, H.B.; Carvalho, R.; Gruetter, R.; Cunha, R. Impact of Caffeine Consumption on Type 2 Diabetes-Induced Spatial Memory Impairment and Neurochemical Alterations in the Hippocampus. Front. Neurosci. 2019, 12, 1015. [Google Scholar] [CrossRef]
- Lizarbe, B.; Soares, A.F.; Larsson, S.; Duarte, J.M.N. Neurochemical Modifications in the Hippocampus, Cortex and Hypothalamus of Mice Exposed to Long-Term High-Fat Diet. Front. Neurosci. 2019, 12, 985. [Google Scholar] [CrossRef]
- Duarte, J.M.N. Metabolism in the Diabetic Brain: Neurochemical Profiling by 1H Magnetic Resonance Spectroscopy. Diabetes Metab. Disord. 2016, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Duarte, J.M.N.; Lei, H.; Mlynárik, V.; Gruetter, R. The neurochemical profile quantified by in vivo1H NMR spectroscopy. NeuroImage 2012, 61, 342–362. [Google Scholar] [CrossRef] [Green Version]
- De La Monte, S.M. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef] [Green Version]
- Barone, E.; Di Domenico, F.; Perluigi, M.; Butterfield, D.A. The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease. Free Radic. Biol. Med. 2021, 176, 16–33. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, D.; Lin, H.; Zhang, Q.; Zheng, L.; Zheng, Y.; Yin, X.; Li, Z.; Liang, S.; Huang, S. Meta-Analysis of Neurochemical Changes Estimated via Magnetic Resonance Spectroscopy in Mild Cognitive Impairment and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 606. [Google Scholar] [CrossRef]
- Song, T.; Song, X.; Zhu, C.; Patrick, R.; Skurla, M.; Santangelo, I.; Green, M.; Harper, D.; Ren, B.; Forester, B.P.; et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 2021, 72, 101503. [Google Scholar] [CrossRef]
- Marjańska, M.; McCarten, J.R.; Hodges, J.S.; Hemmy, L.S.; Terpstra, M. Distinctive Neurochemistry in Alzheimer’s Disease via 7 T In Vivo Magnetic Resonance Spectroscopy. J. Alzheimer’s Dis. 2019, 68, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Degrell, I.; Hellsing, K.; Nagy, E.; Niklasson, F. Amino acid concentrations in cerebrospinal fluid in presenile and senile dementia of Alzheimer type and multi-infarct dementia. Arch. Gerontol. Geriatr. 1989, 9, 123–135. [Google Scholar] [CrossRef]
- Vermeiren, Y.; Le Bastard, N.; Van Hemelrijck, A.; Drinkenburg, W.H.; Engelborghs, S.; De Deyn, P.P. Behavioral correlates of cerebrospinal fluid amino acid and biogenic amine neurotransmitter alterations in dementia. Alzheimer’s Dement. 2013, 9, 488–498. [Google Scholar] [CrossRef]
- Mb, D.W.E.; Beal, M.F.; Mazurek, M.F.; Bird, E.D.; Martin, J.B. A postmortem study of amino acid neurotransmitters in Alzheimer’s disease. Ann. Neurol. 1986, 20, 616–621. [Google Scholar] [CrossRef]
- Perry, T.L.; Yong, V.W.; Bergeron, C.; Ba, S.H.; Jones, K. Amino acids, glutathione, and glutathione transferase activity in the brains of patients with Alzheimer’s disease. Ann. Neurol. 1987, 21, 331–336. [Google Scholar] [CrossRef]
- Pomara, N.; Singh, R.; Deptula, D.; Chou, J.C.; Schwartz, M.B.; LeWitt, P.A. Glutamate and other CSF amino acids in Alzheimer’s disease. Am. J. Psychiatry 1992, 149, 251–254. [Google Scholar] [CrossRef]
- Csernansky, J.G.; Bardgett, M.E.; Sheline, Y.I.; Morris, J.C.; Olney, J.W. CSF excitatory amino acids and severity of illness in Alzheimer’s disease. Neurology 1996, 46, 1715–1720. [Google Scholar] [CrossRef]
- Aquilani, R.; Costa, A.; Maestri, R.; Ramusino, M.C.; Pierobon, A.; Dossena, M.; Solerte, S.B.; Condino, A.M.; Torlaschi, V.; Bini, P.; et al. Mini Nutritional Assessment May Identify a Dual Pattern of Perturbed Plasma Amino Acids in Patients with Alzheimer’s Disease: A Window to Metabolic and Physical Rehabilitation? Nutrients 2020, 12, 1845. [Google Scholar] [CrossRef]
- Chouraki, V.; Preis, S.R.; Yang, Q.; Beiser, A.; Li, S.; Larson, M.G.; Weinstein, G.; Wang, T.J.; Gerszten, R.E.; Vasan, R.S.; et al. Association of amine biomarkers with incident dementia and Alzheimer’s disease in the Framingham Study. Alzheimer’s Dement. 2017, 13, 1327–1336. [Google Scholar] [CrossRef]
- Roysommuti, S.; Wyss, J.M. Perinatal taurine exposure affects adult arterial pressure control. Amino Acids 2014, 46, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Jiang, Z.-L.; Fan, X.-J.; Zhang, L.; Li, X.; Ke, K.-F. Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 2007, 52, 1199–1209. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Bianchi, G.; Martelli, M.; Talerico, T.; Servadei, L.; Zoli, M.; Mariani, E. Plasma amino acid concentrations in patients with amnestic mild cognitive impairment or Alzheimer disease. Am. J. Clin. Nutr. 2004, 80, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Chaney, A.M.; Lopez-Picon, F.R.; Serrière, S.; Wang, R.; Bochicchio, D.; Webb, S.D.; Vandesquille, M.; Harte, M.K.; Georgiadou, C.; Lawrence, C.; et al. Prodromal neuroinflammatory, cholinergic and metabolite dysfunction detected by PET and MRS in the TgF344-AD transgenic rat model of AD: A collaborative multi-modal study. Theranostics 2021, 11, 6644–6667. [Google Scholar] [CrossRef]
- Nilsen, L.H.; Melø, T.M.; Saether, O.; Witter, M.P.; Sonnewald, U.; Sæther, O. Altered neurochemical profile in the McGill-R-Thy1-APP rat model of Alzheimer’s disease: A longitudinalin vivo1H MRS study. J. Neurochem. 2012, 123, 532–541. [Google Scholar] [CrossRef]
- Dedeoglu, A.; Choi, J.-K.; Cormier, K.; Kowall, N.W.; Jenkins, B.G. Magnetic resonance spectroscopic analysis of Alzheimer’s disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain Res. 2004, 1012, 60–65. [Google Scholar] [CrossRef]
- Mlynarik, V.; Cacquevel, M.; Sun-Reimer, L.; Janssens, S.; Cudalbu, C.; Lei, H.; Schneider, B.L.; Aebischer, P.; Gruetter, R. Proton and phosphorus magnetic resonance spectroscopy of a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 31 (Suppl. S3), S87–S99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, D.; Davies, K.; Williams, S. Magnetic resonance spectroscopy in vivo of neurochemicals in a transgenic model of Alzheimer’s disease: A longitudinal study of metabolites, relaxation time, and behavioral analysis in TASTPM and wild-type mice. Magn. Reson. Med. 2013, 69, 944–955. [Google Scholar] [CrossRef]
- Chaney, A.; Bauer, M.; Bochicchio, D.; Smigova, A.; Kassiou, M.; Davies, K.E.; Williams, S.R.; Boutin, H. Longitudinal investigation of neuroinflammation and metabolite profiles in the APPswe × PS 1Δe9 transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2017, 144, 318–335. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Fan, W.; Ma, Z.; Wen, X.; Wang, W.; Wu, Q.; Huang, H. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 2014, 266, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fan, W.; Cai, Y.; Wu, Q.; Mo, L.; Huang, Z.; Huang, H. Protective effects of taurine in traumatic brain injury via mitochondria and cerebral blood flow. Amino Acids 2016, 48, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, Y.; Gu, Y.; Xu, C. Anti-inflammatory mechanism of taurine against ischemic stroke is related to down-regulation of PARP and NF-ϰB. Amino Acids 2011, 42, 1735–1747. [Google Scholar] [CrossRef]
- Junyent, F.; Utrera, J.; Romero, R.; Pallàs, M.; Camins, A.; Duque, D.; Auladell, C. Prevention of epilepsy by taurine treatments in mice experimental model. J. Neurosci. Res. 2009, 87, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9, 435. [Google Scholar] [CrossRef] [Green Version]
- Abuirmeileh, A.N.; Abuhamdah, S.M.; Ashraf, A.; Alzoubi, K.H. Protective effect of caffeine and/or taurine on the 6-hydroxydopamine-induced rat model of Parkinson’s disease: Behavioral and neurochemical evidence. Restor. Neurol. Neurosci. 2021, 39, 149–157. [Google Scholar] [CrossRef]
- Haider, S.; Sajid, I.; Batool, Z.; Madiha, S.; Sadir, S.; Kamil, N.; Liaquat, L.; Ahmad, S.; Tabassum, S.; Khaliq, S. Supplementation of Taurine Insulates Against Oxidative Stress, Confers Neuroprotection and Attenuates Memory Impairment in Noise Stress Exposed Male Wistar Rats. Neurochem. Res. 2020, 45, 2762–2774. [Google Scholar] [CrossRef]
- Jangra, A.; Rajput, P.; Dwivedi, D.; Lahkar, M. Amelioration of Repeated Restraint Stress-Induced Behavioral Deficits and Hippocampal Anomalies with Taurine Treatment in Mice. Neurochem. Res. 2020, 45, 731–740. [Google Scholar] [CrossRef]
- Silva, S.P.; Zago, A.M.; Carvalho, F.B.; Germann, L.; Colombo, G.D.M.; Rahmeier, F.L.; Gutierres, J.M.; Reschke, C.R.; Bagatini, M.D.; Assmann, C.E.; et al. Neuroprotective Effect of Taurine against Cell Death, Glial Changes, and Neuronal Loss in the Cerebellum of Rats Exposed to Chronic-Recurrent Neuroinflammation Induced by LPS. J. Immunol. Res. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gebara, E.; Udry, F.; Sultan, S.; Toni, N. Taurine increases hippocampal neurogenesis in aging mice. Stem Cell Res. 2015, 14, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paula-Lima, A.C.; De Felice, F.G.; Brito-Moreira, J.; Ferreira, S.T. Activation of GABAA receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology 2005, 49, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, H.V.; Yoon, J.H.; Kang, B.R.; Cho, S.M.; Lee, S.; Kim, J.Y.; Kim, J.W.; Cho, Y.; Woo, J.; et al. Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer’s disease. Sci. Rep. 2014, 4, 7467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.J.; Lee, H.-J.; Jeong, Y.J.; Nam, K.R.; Kang, K.J.; Han, S.J.; Lee, K.C.; Lee, Y.J.; Choi, J.Y. Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Santa-María, I.; Hernández, F.; Moreno, F.J.; Avila, J. Taurine, an inducer for tau polymerization and a weak inhibitor for amyloid-beta-peptide aggregation. Neurosci. Lett. 2007, 429, 91–94. [Google Scholar] [CrossRef]
- Caletti, G.; Almeida, F.B.; Agnes, G.; Nin, M.S.; Barros, H.M.T.; Gomez, R. Antidepressant dose of taurine increases mRNA expression of GABAA receptor α2 subunit and BDNF in the hippocampus of diabetic rats. Behav. Brain Res. 2015, 283, 11–15. [Google Scholar] [CrossRef]
- Caletti, G.; Herrmann, A.P.; Pulcinelli, R.R.; Steffens, L.; Morás, A.M.; Vianna, P.; Chies, J.; Moura, D.J.; Barros, H.M.T.; Gomez, R. Taurine counteracts the neurotoxic effects of streptozotocin-induced diabetes in rats. Amino Acids 2017, 50, 95–104. [Google Scholar] [CrossRef]
- Agca, C.A.; Tuzcu, M.; Hayirli, A.; Sahin, K. Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem. Toxicol. 2014, 71, 116–121. [Google Scholar] [CrossRef]
- Rahmeier, F.L.; Zavalhia, L.S.; Tortorelli, L.S.; Huf, F.; Géa, L.P.; Meurer, R.T.; Machado, A.C.; Gomez, R.; Fernandes, M.D.C. The effect of taurine and enriched environment on behaviour, memory and hippocampus of diabetic rats. Neurosci. Lett. 2016, 630, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, X.; Luo, M.; Llah, I.-U.; Wu, P.; Zhang, M.; Zhang, C.; Li, Q.; Wang, Y.; Piao, F. Taurine protects against myelin damage of sciatic nerve in diabetic peripheral neuropathy rats by controlling apoptosis of schwann cells via NGF/Akt/GSK3β pathway. Exp. Cell Res. 2019, 383, 111557. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Serrano, A.M.; Vieira, J.P.P.; Fleischhart, V.; Duarte, J.M.N. Taurine or N-acetylcysteine treatments prevent memory impairment and metabolite profile alterations in the hippocampus of high-fat diet-fed female mice. bioRxiv 2022. [Google Scholar] [CrossRef]
- El Idrissi, A.; El Hilali, F.; Rotondo, S.; Sidime, F. Effects of Taurine Supplementation on Neuronal Excitability and Glucose Homeostasis. Adv. Exp. Med. Biol. 2017, 975, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Santos-Silva, J.C.; Vettorazzi, J.F.; Cotrim, B.B.; Mobiolli, D.D.M.; Boschero, A.C.; Carneiro, E.M. Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic beta-cells. Amino Acids 2012, 43, 1791–1801. [Google Scholar] [CrossRef]
- Drabkova, P.; Sanderova, J.; Kovarik, J.; Kandar, R. An Assay of Selected Serum Amino Acids in Patients with Type 2 Diabetes Mellitus. Adv. Clin. Exp. Med. 2015, 24, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Olsen, T.; Vinknes, K.J.; Refsum, H.; Gulseth, H.L.; Birkeland, K.I.; Drevon, C.A. Plasma Sulphur-Containing Amino Acids, Physical Exercise and Insulin Sensitivity in Overweight Dysglycemic and Normal Weight Normoglycemic Men. Nutrients 2018, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Anuradha, C.V.; Balakrishnan, S.D. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can. J. Physiol. Pharmacol. 1999, 77, 10588478. [Google Scholar] [CrossRef]
- Nakaya, Y.; Minami, A.; Harada, N.; Sakamoto, S.; Niwa, Y.; Ohnaka, M. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am. J. Clin. Nutr. 2000, 71, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Nandhini, A.T.A.; Anuradha, C.V. Taurine modulates kallikrein activity and glucose metabolism in insulin resistant rats. Amino Acids 2002, 22, 27–38. [Google Scholar] [CrossRef]
- Camargo, R.L.; Branco, R.C.S.; De Rezende, L.F.; Vettorazzi, J.F.; Borck, P.C.; Boschero, A.C.; Carneiro, E.M. The Effect of Taurine Supplementation on Glucose Homeostasis: The Role of Insulin-Degrading Enzyme. Adv. Exp. Med. Biol. 2015, 803, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Borck, P.C.; Vettorazzi, J.F.; Branco, R.C.S.; Batista, T.M.; Santos-Silva, J.C.; Nakanishi, V.Y.; Boschero, A.C.; Ribeiro, R.A.; Carneiro, E.M. Taurine supplementation induces long-term beneficial effects on glucose homeostasis in ob/ob mice. Amino Acids 2018, 50, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Vettorazzi, J.F.; Ribeiro, R.A.; Borck, P.C.; Branco, R.C.S.; Soriano, S.; Merino, B.; Boschero, A.C.; Nadal, A.; Quesada, I.; Carneiro, E.M. The bile acid TUDCA increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cells. Metabolism 2016, 65, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Schaffer, S.W.; Azuma, J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids 2011, 42, 1529–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brøns, C.; Spohr, C.; Storgaard, H.; Dyerberg, J.; Vaag, A.A. Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes mellitus. Eur. J. Clin. Nutr. 2004, 58, 1239–1247. [Google Scholar] [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Barbaresko, J.; Lang, A.; Maalmi, H.; Rathmann, W.; Roden, M.; Herder, C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: Umbrella review of meta-analyses of prospective studies. Diabetology 2021, 65, 275–285. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Gustafson, D.R.; Hardy, J. The genetic architecture of Alzheimer’s disease: Beyond APP, PSENs and APOE. Neurobiol. Aging 2012, 33, 437–456. [Google Scholar] [CrossRef] [Green Version]
- Albanese, E.; Launer, L.J.; Egger, M.; Prince, M.; Giannakopoulos, P.; Wolters, F.J.; Egan, K. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2017, 8, 165–178. [Google Scholar] [CrossRef]
- Qizilbash, N.; Gregson, J.; Johnson, M.; Pearce, N.; Douglas, I.; Wing, K.; Evans, S.; Pocock, S.J. BMI and risk of dementia in two million people over two decades: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015, 3, 431–436. [Google Scholar] [CrossRef]
- Sun, S.; He, D.; Luo, C.; Lin, X.; Wu, J.; Yin, X.; Jia, C.; Pan, Q.; Dong, X.; Zheng, F.; et al. Metabolic Syndrome and Its Components Are Associated with Altered Amino Acid Profile in Chinese Han Population. Front. Endocrinol. 2022, 12, 795044. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Li, Y.-Z.; Li, R.; Lan, L.; Li, C.-L.; Huang, M.; Shi, D.; Feng, R.-N.; Sun, C.-H. Dietary Sulfur-Containing Amino Acids Are Associated with Higher Prevalence of Overweight/Obesity in Northern Chinese Adults, an Internet-Based Cross-Sectional Study. Ann. Nutr. Metab. 2018, 73, 44–53. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiee, Z.; García-Serrano, A.M.; Duarte, J.M.N. Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes. Nutrients 2022, 14, 1292. https://doi.org/10.3390/nu14061292
Rafiee Z, García-Serrano AM, Duarte JMN. Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes. Nutrients. 2022; 14(6):1292. https://doi.org/10.3390/nu14061292
Chicago/Turabian StyleRafiee, Zeinab, Alba M. García-Serrano, and João M. N. Duarte. 2022. "Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes" Nutrients 14, no. 6: 1292. https://doi.org/10.3390/nu14061292