Indole-3-Carbinol Inhibits the Growth of Endometriotic Lesions by Suppression of Microvascular Network Formation
Abstract
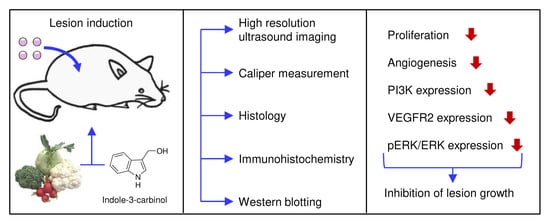
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vaginal Lavage
2.3. Isolation of Uterine Tissue Samples
2.4. Induction of Endometriotic Lesions
2.5. High-Resolution Ultrasound Imaging and Analysis
2.6. Histology and Immunohistochemistry
2.7. Western Blot Analysis
2.8. Experimental Protocol
2.9. Statistics
3. Results
3.1. Effect of I3C on the Development of Endometriotic Lesions
3.2. Effect of I3C on Proliferation and Angiogenesis within Endometriotic Lesions
3.3. Effect of I3C on the Protein Expression of Endometriotic Lesions
3.4. Effect of I3C on the Proliferation and Microvessel Density within the Repoductive Organs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Missmer, S.A.; Cramer, D.W. The epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 2003, 30, 1–19. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D. The modern role of reproductive surgery. Clin. Obstet. Gynecol. 2011, 54, 710–719. [Google Scholar] [CrossRef]
- Armour, M.; Lawson, K.; Wood, A.; Smith, C.A.; Abbott, J. The cost of illness and economic burden of endometriosis and chronic pelvic pain in Australia: A national online survey. PLoS ONE 2019, 14, e0223316. [Google Scholar] [CrossRef] [Green Version]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafique, S.; Decherney, A.H. Medical Management of Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 485–496. [Google Scholar] [CrossRef]
- Becker, C.M.; Gattrell, W.T.; Gude, K.; Singh, S.S. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. Fertil. Steril. 2017, 108, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Salinaro, A.T.; Raffone, E.; Genovese, T.; et al. Autophagy and Mitophagy Promotion in a Rat Model of Endometriosis. Int. J. Mol. Sci. 2021, 22, 5074. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; D’amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355–31366. [Google Scholar] [CrossRef]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Sato, E.; Furue, A.; Nawaz, A.; Hatta, H.; Fukushi, Y.; Wada, S.; Tobe, K.; et al. CD206+ macrophage is an accelerator of endometriotic-like lesion via promoting angiogenesis in the endometriosis mouse model. Sci. Rep. 2021, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.S.; Han, S.J. Endometriosis-Associated Angiogenesis and Anti-angiogenic Therapy for Endometriosis. Front. Glob. Womens Health 2022, 3, 856316. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, L.; Noventa, M.; Ciebiera, M.; Magliarditi, M.; Sleiman, Z.; Karaman, E.; Catena, U.; Salvaggio, C.; Falzone, G.; Garzon, S. Phytotherapy in endometriosis: An up-to-date review. J. Complement. Integr. Med. 2020, 17, 20190084. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Schwender, C.; Scheuer, C.; Vollmar, B.; Menger, M.D. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum. Reprod. 2008, 23, 2308–2318. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Lui, W.T.; Chu, C.Y.; Ng, P.S.; Wang, C.C.; Rogers, M.S. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum. Reprod. 2009, 24, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Laschke, M.W.; van Oijen, A.E.V.; Körbel, C.; Scheuer, C.; Menger, M.D. In vitro and in vivo evaluation of the anti-angiogenic actions of 4-hydroxybenzyl alcohol. Br. J. Pharmacol. 2011, 163, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Liu, Y.; Han, J.; Zai, D.; Ji, M.; Cheng, W.; Xu, L.; Yang, L.; He, M.; Ni, J.; et al. Puerarin suppresses invasion and vascularization of endometriosis tissue stimulated by 17beta-estradiol. PLoS ONE 2011, 6, e25011. [Google Scholar]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Man, G.C.W.; Wang, C.C. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011, 96, 1021–1028. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Hu, Y.Y.; Wang, H.; Zhang, C.J. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int. J. Mol. Med. 2011, 27, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cao, H.; Hu, Y.Y.; Wang, H.; Zhang, C.J. Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum. Reprod. 2012, 27, 1735–1744. [Google Scholar]
- Rudzitis-Auth, J.; Menger, M.D.; Laschke, M.W. Resveratrol is a potent inhibitor of vascularization and cell proliferation in experimental endometriosis. Hum. Reprod. 2013, 28, 1339–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderton, M.J.; Manson, M.M.; Verschoyle, R.D.; Gescher, A.; Lamb, J.H.; Farmer, P.B.; Steward, W.P.; Williams, M.L. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res. 2004, 10, 5233–5241. [Google Scholar] [CrossRef]
- Wu, H.-T.; Lin, S.-H.; Chen, Y.-H. Inhibition of cell proliferation and in vitro markers of angiogenesis by indole-3-carbinol, a major indole metabolite present in cruciferous vegetables. J. Agric. Food Chem. 2005, 53, 5164–5169. [Google Scholar] [CrossRef]
- Kunimasa, K.; Kobayashi, T.; Sugiyama, S.; Kaji, K.; Ohta, T. Indole-3-carbinol suppresses tumor-induced angiogenesis by inhibiting tube formation and inducing apoptosis. Biosci. Biotechnol. Biochem. 2008, 72, 2243–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, F.; Chen, D.Z.; Liu, K.; Sepkovic, D.W.; Bradlow, H.L.; Auborn, K. Anti-estrogenic activities of indole-3-carbinol in cervical cells: Implication for prevention of cervical cancer. Anticancer Res. 1999, 19, 1673–1680. [Google Scholar] [PubMed]
- Meng, Q.; Yuan, F.; Goldberg, I.D.; Rosen, E.M.; Auborn, K.; Fan, S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J. Nutr. 2000, 130, 2927–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudzitis-Auth, J.; Christoffel, A.; Menger, M.D.; Laschke, M.W. Targeting sphingosine kinase-1 with the low MW inhibitor SKI-5C suppresses the development of endometriotic lesions in mice. Br. J. Pharmacol. 2021, 178, 4104–4118. [Google Scholar] [CrossRef]
- Laschke, M.W.; Körbel, C.; Rudzitis-Auth, J.; Gashaw, I.; Reinhardt, M.; Hauff, P.; Zollner, T.M.; Menger, M.D. High-resolution ultrasound imaging: A novel technique for the noninvasive in vivo analysis of endometriotic lesion and cyst formation in small animal models. Am. J. Pathol. 2010, 176, 585–593. [Google Scholar] [CrossRef]
- Rudzitis-Auth, J.; Nenicu, A.; Nickels, R.M.; Menger, M.D.; Laschke, M.W. Estrogen Stimulates Homing of Endothelial Progenitor Cells to Endometriotic Lesions. Am. J. Pathol. 2016, 186, 2129–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, C.M.; Rohwer, N.; Funakoshi, T.; Cramer, T.; Bernhardt, W.; Birsner, A.; Folkman, J.; D’Amato, R.J. 2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am. J. Pathol. 2008, 172, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Luo, J.; Rana, J.S.; Laham, R.; Sellke, F.W.; Li, J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc. Res. 2006, 69, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa-Masuda, A.; Terai, K. ERK activation in endothelial cells is a novel marker during neovasculogenesis. Genes Cells 2016, 21, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auborn, K.J.; Fan, S.; Rosen, E.M.; Goodwin, L.; Chandraskaren, A.; Williams, D.E.; Chen, D.; Carter, T.H. Indole-3-carbinol is a negative regulator of estrogen. J. Nutr. 2003, 133, 2470S–2475S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groothuis, P.; Nap, A.; Winterhager, E.; Grümmer, R. Vascular development in endometriosis. Angiogenesis 2005, 8, 147–156. [Google Scholar] [CrossRef]
- Kapoor, R.; Stratopoulou, C.A.; Dolmans, M.-M. Pathogenesis of Endometriosis: New Insights into Prospective Therapies. Int. J. Mol. Sci. 2021, 22, 11700. [Google Scholar] [CrossRef]
- Lu, H.-F.; Tung, W.-L.; Yang, J.-S.; Huang, F.-M.; Lee, C.-S.; Huang, Y.-P.; Liao, W.-Y.; Chen, Y.-L.; Chung, J.-G. In vitro suppression of growth of murine WEHI-3 leukemia cells and in vivo promotion of phagocytosis in a leukemia mice model by indole-3-carbinol. J. Agric. Food Chem. 2012, 60, 7634–7643. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Reed, G.A.; Peterson, K.S.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. A phase I study of indole-3-carbinol in women: Tolerability and effects. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Souli, E.; Machluf, M.; Morgenstern, A.; Sabo, E.; Yannai, S. Indole-3-carbinol (I3C) exhibits inhibitory and preventive effects on prostate tumors in mice. Food Chem. Toxicol. 2008, 46, 863–870. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Chen, C.; Chen, Z.; Xu, Y.; Wang, Y.; Xiao, B.-K.; Chen, S.-M.; Tao, Z.-Z. Indole-3-carbinol inhibits cell proliferation and induces apoptosis in Hep-2 laryngeal cancer cells. Oncol. Rep. 2013, 30, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.M.; Park, S.-H.; Nam, M.J. Anticarcinogenic effect of indole-3-carbinol (I3C) on human hepatocellular carcinoma SNU449 cells. Hum. Exp. Toxicol. 2019, 38, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, H.; Lee, C.; Park, S.-H.; Nam, M. Indole-3-carbinol inhibits the proliferation of colorectal carcinoma LoVo cells through activation of the apoptotic signaling pathway. Hum. Exp. Toxicol. 2021, 40, 2099–2112. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum. Reprod. Update 2018, 24, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Kunimasa, K.; Kobayashi, T.; Kaji, K.; Ohta, T. Antiangiogenic effects of indole-3-carbinol and 3,3′-diindolylmethane are associated with their differential regulation of ERK1/2 and Akt in tube-forming HUVEC. J. Nutr. 2010, 140, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.-L.; Shih, C.-K.; Chang, H.-P.; Chen, Y.-H. Antiangiogenic activity of indole-3-carbinol in endothelial cells stimulated with activated macrophages. Food Chem. 2012, 134, 811–820. [Google Scholar] [CrossRef]
- Hajra, S.; Patra, A.R.; Basu, A.; Saha, P.; Bhattacharya, S. Indole-3-Carbinol (I3C) enhances the sensitivity of murine breast adenocarcinoma cells to doxorubicin (DOX) through inhibition of NF-kappabeta, blocking angiogenesis and regulation of mitochondrial apoptotic pathway. Chem. Biol. Interact. 2018, 290, 19–36. [Google Scholar] [CrossRef]
- Madanes, D.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I.; Ricci, A.G. PI3K/AKT pathway is altered in the endometriosis patient’s endometrium and presents differences according to severity stage. Gynecol. Endocrinol. 2020, 36, 436–440. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef]
- Hung, W.-C.; Chang, H.-C. Indole-3-carbinol inhibits Sp1-induced matrix metalloproteinase-2 expression to attenuate migration and invasion of breast cancer cells. J. Agric. Food Chem. 2009, 57, 76–82. [Google Scholar] [CrossRef]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. PI3K: Downstream AKTion blocks apoptosis. Cell 1997, 88, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Biersack, B.; Li, Y.; Kong, D.; Bao, B.; Schobert, R.; B Padhye, S.; H Sarkar, F. Targeted regulation of PI3K/Akt/mTOR/NF-kappaB signaling by indole compounds and their derivatives: Mechanistic details and biological implications for cancer therapy. Anticancer Agents Med. Chem. 2013, 13, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Faes, S.; Dormond, O. PI3K and AKT: Unfaithful Partners in Cancer. Int. J. Mol. Sci. 2015, 16, 21138–21152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arlier, S.; Murk, W.; Guzeloglu-Kayisli, O.; Semerci, N.; Larsen, K.; Tabak, M.S.; Arici, A.; Schatz, F.; Lockwood, C.J.; Kayisli, U.A. The extracellular signal-regulated kinase 1/2 triggers angiogenesis in human ectopic endometrial implants by inducing angioblast differentiation and proliferation. Am. J. Reprod. Immunol. 2017, 78, e12760. [Google Scholar] [CrossRef]
- Arlier, S.; Murk, W.; Guzeloglu-Kayisli, O.; Semerci, N.; Larsen, K.; Tabak, M.S.; Arici, A.; Schatz, F.; Lockwood, C.J.; Kayisli, U.A. MAPK/ERK signal pathway involved expression of COX-2 and VEGF by IL-1β induced in human endometriosis stromal cells in vitro. Int. J. Clin. Exp. Pathol. 2013, 6, 2129–2136. [Google Scholar]
- Lai, Z.Z.; Yang, H.L.; Ha, S.Y.; Chang, K.K.; Mei, J.; Zhou, W.J.; Qiu, X.M.; Wang, X.Q.; Zhu, R.; Li, D.J.; et al. Cyclooxygenase-2 in Endometriosis. Int. J. Biol. Sci. 2019, 15, 2783–2797. [Google Scholar] [CrossRef] [Green Version]
- Nouriemamzaden, F.; Word, B.; Cotton, E.; Hawkins, A.; Littlejohn, K.; Moore, R.; Miranda-Carbon, G.; Orish, C.N.; Lyn-Cook, B. Modulation of Estrogen α and Progesterone Receptors in Triple Negative Breast Cancer Cell Lines: The Effects of Vorinostat and Indole-3-Carbinol In Vitro. Anticancer Res. 2020, 40, 3669–3683. [Google Scholar] [CrossRef]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of estrogen receptor-beta in endometriosis. Semin. Reprod. Med. 2012, 30, 39–45. [Google Scholar]
- Chen, I.; Safe, S.; Bjeldanes, L. Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer cells. Biochem. Pharmacol. 1996, 51, 1069–1076. [Google Scholar] [CrossRef]
- Lavallee, T.M.; Zhan, X.H.; Herbstritt, C.J.; Kough, E.C.; Green, S.J.; Pribluda, V.S. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002, 62, 3691–3697. [Google Scholar]
- Bell, M.C.; Crowley-Nowick, P.; Bradlow, H.L.; Sepkovic, D.W.; Schmidt-Grimminger, D.; Howell, P.; Mayeaux, E.; Tucker, A.; Turbat-Herrera, E.A.; Mathis, J.M. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol. Oncol. 2000, 78, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, C.A.; Chow, H.H.S.; Wertheim, B.C.; Roe, D.J.; Stopeck, A.; Maskarinec, G.; Altbach, M.; Chalasani, P.; Huang, C.; Strom, M.B.; et al. A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat 2017, 165, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E. Indoles Derived from Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3′-Diindolylmethane. Front. Nutr. 2021, 8, 734334. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudzitis-Auth, J.; Becker, M.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Indole-3-Carbinol Inhibits the Growth of Endometriotic Lesions by Suppression of Microvascular Network Formation. Nutrients 2022, 14, 4940. https://doi.org/10.3390/nu14224940
Rudzitis-Auth J, Becker M, Scheuer C, Menger MD, Laschke MW. Indole-3-Carbinol Inhibits the Growth of Endometriotic Lesions by Suppression of Microvascular Network Formation. Nutrients. 2022; 14(22):4940. https://doi.org/10.3390/nu14224940
Chicago/Turabian StyleRudzitis-Auth, Jeannette, Madeleine Becker, Claudia Scheuer, Michael D. Menger, and Matthias W. Laschke. 2022. "Indole-3-Carbinol Inhibits the Growth of Endometriotic Lesions by Suppression of Microvascular Network Formation" Nutrients 14, no. 22: 4940. https://doi.org/10.3390/nu14224940