Temporal Association of Total Serum Cholesterol and Pancreatic Cancer Incidence
Abstract
:1. Introduction
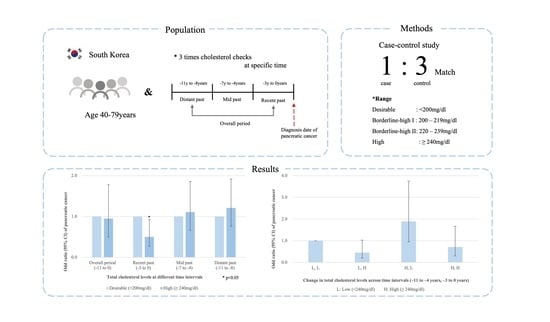
2. Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 2140–2141. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Wolfgang, C.L.; Herman, J.M.; Laheru, D.A.; Klein, A.P.; Erdek, M.A.; Fishman, E.K.; Hruban, R.H. Recent progress in pancreatic cancer. CA Cancer J. Clin. 2013, 63, 318–348. [Google Scholar] [CrossRef] [Green Version]
- Tornberg, S.A.; Holm, L.E.; Carstensen, J.M.; Eklund, G.A. Cancer incidence and cancer mortality in relation to serum cholesterol. J. Natl. Cancer Inst. 1989, 81, 1917–1921. [Google Scholar] [CrossRef]
- Law, M.R.; Thompson, S.G. Low serum cholesterol and the risk of cancer: An analysis of the published prospective studies. Cancer Causes Control 1991, 2, 253–261. [Google Scholar] [CrossRef]
- Mamtani, R.; Lewis, J.D.; Scott, F.I.; Ahmad, T.; Goldberg, D.S.; Datta, J.; Yang, Y.X.; Boursi, B. Disentangling the Association between Statins, Cholesterol, and Colorectal Cancer: A Nested Case-Control Study. PLoS Med. 2016, 13, e1002007. [Google Scholar] [CrossRef]
- Schatzkin, A.; Hoover, R.N.; Taylor, P.R.; Ziegler, R.G.; Carter, C.L.; Albanes, D.; Larson, D.B.; Licitra, L.M. Site-specific analysis of total serum cholesterol and incident cancer in the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Cancer Res. 1988, 48, 452–458. [Google Scholar] [PubMed]
- Guillaumond, F.; Bidaut, G.; Ouaissi, M.; Servais, S.; Gouirand, V.; Olivares, O.; Lac, S.; Borge, L.; Roques, J.; Gayet, O.; et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, 2473–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Chen, G.; Wu, W.M.; Zhou, L.; You, L.; Zhang, T.P.; Zhao, Y.P. Metabolic syndrome components and risk factors for pancreatic adenocarcinoma: A case-control study in China. Digestion 2012, 86, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland). Cancer Causes Control 2002, 13, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D.; Stocks, T.; Jonsson, H.; Lindkvist, B.; Bjorge, T.; Concin, H.; Almquist, M.; Haggstrom, C.; Engeland, A.; Ulmer, H.; et al. Metabolic factors and the risk of pancreatic cancer: A prospective analysis of almost 580,000 men and women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2307–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitahara, C.M.; Berrington de Gonzalez, A.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef] [Green Version]
- Kuzmickiene, I.; Everatt, R.; Virviciute, D.; Tamosiunas, A.; Radisauskas, R.; Reklaitiene, R.; Milinaviciene, E. Smoking and other risk factors for pancreatic cancer: A cohort study in men in Lithuania. Cancer Epidemiol. 2013, 37, 133–139. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.Y.; Chlebowski, R.T.; Vitolins, M.Z.; Wassertheil-Smoller, S.; Rohan, T.E. Serum lipids and risk of obesity-related cancers in postmenopausal women. Cancer Causes Control 2018, 29, 13–24. [Google Scholar] [CrossRef]
- Berrington de Gonzalez, A.; Yun, J.E.; Lee, S.Y.; Klein, A.P.; Jee, S.H. Pancreatic cancer and factors associated with the insulin resistance syndrome in the Korean cancer prevention study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Kim, S.-W.; Yu, I.; Lee, I.J.; Park, M.-S.; Hwang, S.H.; Yang, H.K.; Jeong, W.K.; Lee, S.S.; Kim, J.H.; Choi, J.Y. Korean clinical practice guideline for pancreatic cancer 2021: A summary of evidence-based, multi-disciplinary diagnostic and therapeutic approaches. Pancreatology 2021, 21, 1326–1341. [Google Scholar]
- Seo, H.J.; Oh, I.-H.; Yoon, S.-J. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac. J. Cancer Prev. 2012, 13, 6163–6168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health. ATP III Guidelines at-a-Glance Quick Desk Reference; NIH: Bethesda, MD, USA, 2001; No. 01–3305. [Google Scholar]
- Keum, N.; Ha, K.H.; Bao, Y.; Chung, M.J.; Kim, H.C.; Giovannucci, E.L. Long-term patterns of fasting blood glucose levels and pancreatic cancer incidence. Cancer Causes Control 2018, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Strohmaier, S.; Edlinger, M.; Manjer, J.; Stocks, T.; Bjorge, T.; Borena, W.; Haggstrom, C.; Engeland, A.; Nagel, G.; Almquist, M.; et al. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS ONE 2013, 8, e54242. [Google Scholar] [CrossRef] [Green Version]
- Ansary-Moghaddam, A.; Huxley, R.; Barzi, F.; Lawes, C.; Ohkubo, T.; Fang, X.; Jee, S.H.; Woodward, M.; Asia Pacific Cohort Studies, C. The effect of modifiable risk factors on pancreatic cancer mortality in populations of the Asia-Pacific region. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2435–2440. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.-Y.; Boursi, B.; Mamtani, R.; Yang, Y.-X. Total Serum Cholesterol and Pancreatic Cancer: A Nested Case–Control Study. Cancer Epidemiol. Prev. Biomark. 2019, 28, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Gabitova, L.; Gorin, A.; Astsaturov, I. Molecular pathways: Sterols and receptor signaling in cancer. Clin. Cancer Res. 2014, 20, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Song, B.L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Razidlo, G.L.; Burton, K.M.; McNiven, M.A. Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42. J. Biol. Chem. 2018, 293, 11143–11153. [Google Scholar] [CrossRef] [Green Version]
- Mormile, R. Total Serum Cholesterol and Pancreatic Cancer Risk: What Is the Link? Pathol. Oncol. Res. 2020, 26, 1361. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Han, K.; Hong, J.Y.; Park, Y.S.; Hur, K.Y.; Kang, G.; Park, J.O. Changes in Metabolic Syndrome Status are Associated With Altered Risk of Pancreatic Cancer: A Nationwide Cohort Study. Gastroenterology 2022, 162, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-h.; Jang, J.-Y.; Kang, J.S.; Kim, J.R.; Han, Y.; Kim, E.; Kwon, W.; Kim, S.-W. Recent treatment patterns and survival outcomes in pancreatic cancer according to clinical stage based on single-center large-cohort data. Ann. Hepato-Biliary-Pancreat. Surg. 2018, 22, 386–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muttillo, E.M.; Ciardi, A.; Troiano, R.; Saullo, P.; Masselli, G.; Guida, M.; Tortora, A.; Sperduti, I.; Marinello, G.; Chirletti, P. Pancreatic ductal adenocarcinoma and distal cholangiocarcinoma: A proposal of preoperative diagnostic score for differential diagnosis. World J. Surg. Oncol. 2021, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, M.; Sun, C.; Qu, G.; Shi, T.; Min, M.; Wu, Y.; Sun, Y. Statin Use and Risk of Pancreatic Cancer: An Updated Meta-analysis of 26 Studies. Pancreas 2019, 48, 142–150. [Google Scholar] [CrossRef] [PubMed]

| Cumulative Total Cholesterol Levels (mg/dL) | ||||
|---|---|---|---|---|
| Characteristics 2 | Desirable (<200) | Borderline-High I (200–219) | Borderline-High II (220–239) | High (≥240) |
| No of controls | 340 | 171 | 92 | 42 |
| Average total cholesterol levels (mg/dL) | ||||
| Overall period (−11 to 0 years) | 176.4 (14.4) | 209.7 (5.6) | 228.6 (5.4) | 261.9 (13.8) |
| −3 to 0 years | 174.3 (21.7) | 207.4 (21.0) | 230.3 (23.8) | 264.0 (24.7) |
| −7 to −4 years | 174.8 (21.5) | 209.0 (18.8) | 226.2 (18.2) | 255.1 (18.2) |
| −11 to −8 years | 182.4 (24.9) | 211.3 (22.0) | 230.8 (31.7) | 263.7 (29.3) |
| Age at the index date (years) | 57.3 (9.5) | 57.6 (9.1) | 56.7 (9.2) | 58.7 (9.2) |
| Women (%) | 34.5 | 41.1 | 45.7 | 68.0 |
| Body mass index (kg/m2) | 23.9 (2.8) | 24.3 (2.8) | 24.3 (2.8) | 24.8 (2.5) |
| Ever smokers (%) | 48.8 | 52.0 | 46.5 | 30.5 |
| Regular physical activity (%) | 19.7 | 18.7 | 19.0 | 16.7 |
| Alcohol consumption (g/day) | 60.2 | 58.4 | 52.0 | 52.9 |
| Statin use (%) | 21.7 | 32.5 | 42.6 | 44.3 |
| Time Interval of Total Cholesterol Measurement | Total Cholesterol Levels (mg/dL) | No. of Cases/Controls | Odds Ratio (95% CI) For Pancreatic Cancer | ||
|---|---|---|---|---|---|
| Age and Sex Stratified | Multivariable 1 | Multivariable 1 + Statin Use | |||
| Overall period (−11 to 0 years) | Desirable (<200) | 119/340 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Borderline-high I (200–219) | 54/171 | 0.90 (0.62,1.31) | 0.89 (0.62, 1.30) | 0.87 (0.60, 1.26) | |
| Borderline-high II (220–239) | 27/92 | 0.84 (0.52,1.35) | 0.83 (0.52, 1.34) | 0.79 (0.49, 1.27) | |
| High (≥240) | 15/42 | 1.02 (0.55,1.90) | 1.01 (0.54, 1.89) | 0.95 (0.50, 1.78) | |
| p for trend | 0.62 | 0.58 | 0.41 | ||
| Recent past (−3 to 0 years) | Desirable (<200) | 131/367 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Borderline-high I (200–219) | 41/124 | 0.92 (0.62, 1.38) | 0.93 (0.62, 1.39) | 0.94 (0.62, 1.40) | |
| Borderline-high II (220–239) | 28/81 | 1.01 (0.63, 1.61) | 1.00 (0.62, 1.60) | 1.02 (0.64, 1.64) | |
| High (≥240) | 14/73 | 0.53 (0.29, 0.98) | 0.52 (0.28, 0.95) | 0.50 (0.27, 0.93) | |
| p for trend | 0.12 | 0.1 | 0.09 | ||
| Mid past (−7 to −4 years) | Desirable (<200) | 119/359 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Borderline-high I (200–219) | 41/139 | 0.89 (0.59, 1.34) | 0.89 (0.59, 1.33) | 0.89 (0.59, 1.33) | |
| Borderline-high II (220–239) | 30/81 | 1.12 (0.70, 1.78) | 1.13 (0.71, 1.80) | 1.12 (0.70, 1.80) | |
| High (≥240) | 25/66 | 1.14 (0.69, 1.90) | 1.12 (0.68, 1.87) | 1.11 (0.66, 1.86) | |
| p for trend | 0.66 | 0.69 | 0.72 | ||
| Distant past (−11 to −8 years) | Desirable (<200) | 107/325 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Borderline-high I (200–219) | 43/134 | 0.97 (0.65, 1.47) | 0.95 (0.63, 1.43) | 0.94 (0.63, 1.43) | |
| Borderline-high II (220–239) | 30/101 | 0.90 (0.56, 1.43) | 0.87 (0.54, 1.39) | 0.87 (0.54, 1.38) | |
| High (≥240) | 35/85 | 1.26 (0.80, 1.99) | 1.21 (0.77, 1.92) | 1.21 (0.76, 1.92) | |
| p for trend | 0.55 | 0.7 | 0.71 | ||
| Patterns 1 of Total Cholesterol Levels across Time Intervals (−11 to −4 Years, −3 to 0 Years) | No of Cases/Control | Age and Sex Stratified | Multivariable 2 | Multivariable 2 + Statin Use | |
|---|---|---|---|---|---|
| Consistently low cholesterol | (L, L) | 185/550 | 1 (reference) | 1 (reference) | 1 (reference) |
| Recent-onset hypercholesterolemia | (L, H) | 7/45 | 0.46 (0.20, 1.05) | 0.47 (0.21, 1.06) | 0.45 (0.20, 1.03) |
| Recent-resolved hypercholesterolemia | (H, L) | 16/22 | 2.11 (1.09, 4.08) | 2.07 (1.07, 4.02) | 1.89 (0.95, 3.75) |
| Consistent hypercholesterolemia | (H, H) | 7/28 | 0.74 (0.32, 1.73) | 0.73 (0.31, 1.71) | 0.71 (0.30, 1.66) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.-L.; Khil, J.; Hong, S.; Lee, D.H.; Ha, K.H.; Keum, N.; Kim, H.C.; Giovannucci, E.L. Temporal Association of Total Serum Cholesterol and Pancreatic Cancer Incidence. Nutrients 2022, 14, 4938. https://doi.org/10.3390/nu14224938
Wang Q-L, Khil J, Hong S, Lee DH, Ha KH, Keum N, Kim HC, Giovannucci EL. Temporal Association of Total Serum Cholesterol and Pancreatic Cancer Incidence. Nutrients. 2022; 14(22):4938. https://doi.org/10.3390/nu14224938
Chicago/Turabian StyleWang, Qiao-Li, Jaewon Khil, SungEun Hong, Dong Hoon Lee, Kyoung Hwa Ha, NaNa Keum, Hyeon Chang Kim, and Edward L. Giovannucci. 2022. "Temporal Association of Total Serum Cholesterol and Pancreatic Cancer Incidence" Nutrients 14, no. 22: 4938. https://doi.org/10.3390/nu14224938