The Relationship between Serum Adipokines, miR-222-3p, miR-103a-3p and Glucose Regulation in Pregnancy and Two to Three Years Post-Delivery in Women with Gestational Diabetes Mellitus Adhering to Mediterranean Diet Recommendations
Abstract
:1. Introduction
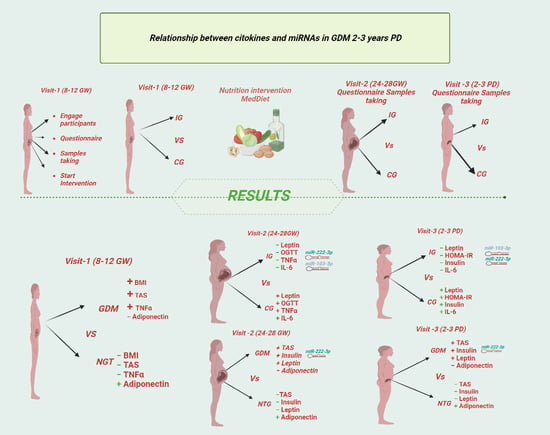
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Subjects
2.4. Data Collection
2.4.1. Demographic Data
2.4.2. Clinical Data
2.4.3. Lifestyle Assessment
2.4.4. Biochemical and miRNA Analysis
Sample Collection and Biochemical Measurements
Adipokine and Cytokine Analysis
miRNA Isolation and Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filardi, T.; Catanzaro, G.; Mardente, S.; Zicari, A.; Santangelo, C.; Lenzi, A.; Morano, S.; Ferretti, E. Non-Coding RNA: Role in Gestational Diabetes Pathophysiology and Complications. Int. J. Mol. Sci. 2020, 21, 4020. [Google Scholar] [CrossRef] [PubMed]
- Abell, S.K.; De Courten, B.; Boyle, J.A.; Teede, H.J. Inflammatory and Other Biomarkers: Role in Pathophysiology and Prediction of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2015, 16, 13442–13473. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007, 30 (Suppl. S2), S112–S119. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Gracia, T.; Duran, A.; Fuentes, M.; Rubio, M.A.; Runkle, I.; Carrera, E.F.; Torrejón, M.J.; Bordiú, E.; Valle, L.D.; García de la Torre, N.; et al. Lifestyle patterns in early pregnancy linked to gestational diabetes mellitus diagnoses when using IADPSG criteria. The St Carlos gestational study. Clin. Nutr. 2016, 35, 699–705. [Google Scholar] [CrossRef]
- Barabash, A.; Valerio, J.D.; Garcia de la Torre, N.; Jimenez, I.; Del Valle, L.; Melero, V.; Assaf-Balut, C.; Fuentes, M.; Bordiu, E.; Duran, A.; et al. TCF7L2 rs7903146 polymorphism modulates the association between adherence to a Mediterranean diet and the risk of gestational diabetes mellitus. Metabol. Open 2020, 8, 100069. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García de la Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Santangelo, C.; Zicari, A.; Mandosi, E.; Scazzocchio, B.; Mari, E.; Morano, S.; Masella, R. Could gestational diabetes mellitus be managed through dietary bioactive compounds? Current knowledge and future perspectives. Br. J. Nutr. 2016, 115, 1129–1144. [Google Scholar] [CrossRef]
- Miehle, K.; Stepan, H.; Fasshauer, M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin. Endocrinol. 2012, 76, 2–11. [Google Scholar] [CrossRef]
- Highman, T.J.; Friedman, J.E.; Huston, L.P.; Wong, W.W.; Catalano, P.M. Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am. J. Obstet. Gynecol. 1998, 178, 1010–1015. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Darbinian, J.; Havel, P.J.; Quesenberry, C.P.; Sridhar, S.; Ehrlich, S.; Ferrara, A. Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care 2013, 36, 3930–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and Insulin Resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef]
- Chen, J.; Tan, B.; Karteris, E.; Zervou, S.; Digby, J.; Hillhouse, E.W.; Vatish, M.; Randeva, H.S. Secretion of adiponectin by human placenta: Differential modulation of adiponectin and its receptors by cytokines. Diabetologia 2006, 49, 1292–1302. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhao, Y.H.; Chen, Y.P.; Yuan, X.L.; Wang, J.; Zhu, H.; Lu, C.M. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: A systematic review and meta-analysis. Sci. World J. 2014, 2014, 926932. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Xing, W.; Xie, L. Regulatory Roles of MicroRNAs in Diabetes. Int. J. Mol. Sci. 2016, 17, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geekiyanage, H.; Rayatpisheh, S.; Wohlschlegel, J.A.; Brown, R., Jr.; Ambros, V. Extracellular microRNAs in human circulation are associated with miRISC complexes that are accessible to anti-AGO2 antibody and can bind target mimic oligonucleotides. Proc. Natl. Acad. Sci. USA 2020, 117, 24213–24223. [Google Scholar] [CrossRef]
- Zhu, H.; Leung, S.W. Identification of microRNA biomarkers in type 2 diabetes: A meta-analysis of controlled profiling studies. Diabetologia 2015, 58, 900–911. [Google Scholar] [CrossRef] [Green Version]
- Gomes, P.R.; Graciano, M.F.; Pantaleão, L.C.; Rennó, A.L.; Rodrigues, S.C.; Velloso, L.A.; Latorraca, M.Q.; Carpinelli, A.R.; Anhê, G.F.; Bordin, S. Long-term disruption of maternal glucose homeostasis induced by prenatal glucocorticoid treatment correlates with miR-29 upregulation. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E109–E120. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Igata, M.; Kondo, T.; Kitano, S.; Takaki, Y.; Hanatani, S.; Sakaguchi, M.; Goto, R.; Senokuchi, T.; Kawashima, J.; et al. Identification of microRNA that represses IRS-1 expression in liver. PLoS ONE 2018, 13, e0191553. [Google Scholar] [CrossRef]
- Luo, M.; Xu, C.; Luo, Y.; Wang, G.; Wu, J.; Wan, Q. Circulating miR-103 family as potential biomarkers for type 2 diabetes through targeting CAV-1 and SFRP4. Acta Diabetol. 2020, 57, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, S.; Muhammad, S.; Ren, Q.; Sun, C. miR-103/107 promote ER stress-mediated apoptosis via targeting the Wnt3a/β-catenin/ATF6 pathway in preadipocytes. J. Lipid Res. 2018, 59, 843–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Dong, J.; Jiang, T.; Shi, Z.; Yu, B.; Zhu, Y.; Chen, D.; Xu, J.; Huo, R.; Dai, J.; et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS ONE 2011, 6, e23925. [Google Scholar] [CrossRef]
- Wander, P.L.; Boyko, E.J.; Hevner, K.; Parikh, V.J.; Tadesse, M.G.; Sorensen, T.K.; Williams, M.A.; Enquobahrie, D.A. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res. Clin. Pract. 2017, 132, 1–9. [Google Scholar] [CrossRef]
- Ibarra, A.; Vega-Guedes, B.; Brito-Casillas, Y.; Wägner, A.M. Diabetes in Pregnancy and MicroRNAs: Promises and Limitations in Their Clinical Application. Noncoding RNA 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Mohany, K.M.; Al Rugaie, O.; Al-Wutayd, O.; Al-Nafeesah, A. Investigation of the levels of circulating miR-29a, miR-122, sestrin 2 and inflammatory markers in obese children with/without type 2 diabetes: A case control study. BMC Endocr. Disord. 2021, 21, 152. [Google Scholar] [CrossRef]
- Harreiter, J.; Simmons, D.; Desoye, G.; Corcoy, R.; Adelantado, J.M.; Devlieger, R.; Van Assche, A.; Galjaard, S.; Damm, P.; Mathiesen, E.R.; et al. IADPSG and WHO 2013 Gestational Diabetes Mellitus Criteria Identify Obese Women with Marked Insulin Resistance in Early Pregnancy. Diabetes Care 2016, 39, e90–e92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, A.F.; Calle-Pascual, A.L. Diabetes Nutrition and Complications Trial: Trends in nutritional pattern between 1993 and 2000 and targets of diabetes treatment in a sample of Spanish people with diabetes. Diabetes Care 2004, 27, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Assaf-Balut, C.; García De La Torre, N.; Fuentes, M.; Durán, A.; Bordiú, E.; Del Valle, L.; Valerio, J.; Jiménez, I.; Herraiz, M.; Izquierdo, N.; et al. A High Adherence to Six Food Targets of the Mediterranean Diet in the Late First Trimester is Associated with a Reduction in the Risk of Materno-Foetal Outcomes: The St. Carlos Gestational Diabetes Mellitus Prevention Study. Nutrients 2018, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project. Jamovi (Version 1.6). 2021. Available online: https://www.jamovi.org (accessed on 1 January 2022).
- Bogdanet, D.; Reddin, C.; Murphy, D.; Doheny, H.C.; Halperin, J.A.; Dunne, F.; O’Shea, P.M. Emerging Protein Biomarkers for the Diagnosis or Prediction of Gestational Diabetes—A Scoping Review. J. Clin. Med. 2021, 10, 1533. [Google Scholar] [CrossRef]
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Choi, W.-Y.; Heo, J.H.; Huh, J.; Kim, G.; Lee, K.-P.; Kwun, H.-J.; Shin, H.-J.; Baek, I.-J.; Hong, E.-J. Progesterone increases blood glucose via hepatic progesterone receptor membrane component 1 under limited or impaired action of insulin. Sci. Rep. 2020, 10, 16316. [Google Scholar] [CrossRef] [PubMed]
- Winzer, C.; Wagner, O.; Festa, A.; Schneider, B.; Roden, M.; Bancher-Todesca, D.; Pacini, G.; Funahashi, T.; Kautzky-Willer, A. Plasma Adiponectin, Insulin Sensitivity, and Subclinical Inflammation in Women With Prior Gestational Diabetes Mellitus. Diabetes Care 2004, 27, 1721–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Zhao, C.; Guo, X.; Ding, H.; Cui, Y.; Shen, R.; Liu, J. Differential expression of microRNAs in omental adipose tissue from gestational diabetes mellitus subjects reveals miR-222 as a regulator of ERα expression in estrogen-induced insulin resistance. Endocrinology 2014, 155, 1982–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiuliis, J.A. MicroRNAs as regulators of metabolic disease: Pathophysiologic significance and emerging role as biomarkers and therapeutics. Int. J. Obes. 2016, 40, 88–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frias, F.T.; Rocha, K.C.E.; de Mendonça, M.; Murata, G.M.; Araujo, H.N.; de Sousa, L.G.O.; de Sousa, É.; Hirabara, S.M.; Leite, N.C.; Carneiro, E.M.; et al. Fenofibrate reverses changes induced by high-fat diet on metabolism in mice muscle and visceral adipocytes. J. Cell Physiol. 2018, 233, 3515–3528. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, S.; Cepparulo, P.; Vinciguerra, A.; Campanile, M.; Esposito, G.; Maruotti, G.M.; Zullo, F.; Annunziato, L.; Pignataro, G. miR-16-5p, miR-103-3p, and miR-27b-3p as Early Peripheral Biomarkers of Fetal Growth Restriction. Front. Pediatr. 2021, 9, 611112. [Google Scholar] [CrossRef]
| Control Group n = 141 | Intervention Group n = 172 | p | |
|---|---|---|---|
| Age (years) | 33.6 ± 4.8 | 34.5 ± 4.7 | 0.112 |
| Race/Ethnicity | |||
| Caucasian | 85 (61.3) | 113 (65.7) | 0.020 |
| Hispanic | 50 (35.5) | 56 (32.6) | |
| Others | 6 (4.2) | 3 (1.8) | |
| Family history of | |||
| Type 2 Diabetes | 31 (21.9) | 49 (28.5) | 0.174 |
| MetS (>2 components) | 26 (18.4) | 42 (24.4) | |
| Previous history of | |||
| - Gestational DM | 7 (5.0) | 6 (3.5) | 0.791 |
| - Miscarriages | 49 (34.7) | 61 (35.5) | |
| Educational status | |||
| Elementary education | 15 (10.6) | 9 (5.2) | 0.109 |
| Secondary School | 67 (47.5) | 73 (42.4) | |
| University Degree | 57 (40.4) | 89 (51.7) | |
| UNK | 2 (1.4) | 1 (0.6) | |
| Employed | 105 (74.5) | 140 (81.4) | 0.510 |
| Number of pregnancies | |||
| Primiparous | 45 (31.9) | 60 (35.1) | 0.214 |
| Second pregnancy | 49 (34.8) | 73 (42.4) | |
| >2 pregnancies | 47 (33.3) | 39 (22.5) | |
| Smoker | |||
| Never | 77 (54.6) | 95 (55.2) | 0.701 |
| Current | 9 (6.4) | 14 (8.1) | |
| Gestational Age (weeks) at baseline | 12.1 ± 0.6 | 12.1 ± 0.5 | 0.838 |
| Pre-pregnancy self-reported body Weight (kg) | 60.0 (53.5–67.0) | 58.2 (54.5–65.9) | 0.744 |
| Pregnancy Body Weight at entry, 8–12 GW (kg) | 61.6 (55.0–69.0) | 60.0 (58.8–68.6) | 0.910 |
| Weight gain at 8–12 GW | 2.0 (0.5–3.8) | 1.7 (0.2–3.1) | 0.053 |
| Pre-pregnancy BMI (kg/m2) | 22.6 (20.4–25.5) | 22.7 (20.5–25.1) | 0.927 |
| Systolic BP/Diastolic BP (mm Hg) | 105 ± 9/63 ± 8 | 107 ± 11/66 ± 9 | 0.415/0.367 |
| Fasting Blood Glucose (mg/dL) | 80.9 ± 6.4 | 80.8 ± 6.2 | 0.917 |
| HbA1c (%) | 5.1 ± 0.1 | 5.2 ± 0.2 | 0.563 |
| Fasting Cholesterol (mg/dL) | 175 ± 32 | 176 ± 27 | 0.725 |
| Fasting Tryglycerides (mg/dL) | 87 ± 39 | 89 ± 56 | 0.767 |
| Fasting Plasma Insulin (µUI/mL) | 12.3 (8.1–28.1) | 11.8 (6.1–21.0) | 0.09 |
| HOMA-IR | 2.6 (1.6–5.5) | 2.3(1.2–4.2) | 0.089 |
| Adiponectin (µg/mL) | 20.4 (14.1–27.6) | 17.7 (13.3–22.5) | 0.081 |
| IL-6 (pg/mL) | 3.0 (1.5–7.3) | 2.0 (0.1–4.9) | 0.071 |
| Leptin (ng/mL) | 7.3 (4.5–13.2) | 6.5 (3.6–10.9) | 0.099 |
| TNF-α (pg/mL) | 2.9 (2.1–4.4) | 2.4 (1.7–3.3) | 0.071 |
| TSH mcUI/mL | 1.84 ± 1.17 | 1.98 ± 1.46 | 0.394 |
| FT4 (pg/dL) | 8.68 ± 1.59 | 8.76 ± 1.35 | 0.656 |
| MEDAS Score | 4.8 ± 1.7 | 5.1 ± 1.61 | 0.096 |
| Physical Activity Score | −1.7 ± 1.1 | −1.9 ± 1.0 | 0.251 |
| Nutrition Score | 0.9 ± 3.3 | 0.6 ± 3.2 | 0.452 |
| GDM at 24–28 GW n (%) | 40 (28.4) | 37 (21.5) | 0.102 |
| GDM n = 77 | NGT n = 236 | p | |
|---|---|---|---|
| Age (years) | 34.9 ± 4.6 | 33.8 ± 4.8 | 0.056 |
| Race/Ethnicity | |||
| Caucasian | 51 (66.2) | 147 (62.3) | 0.132 |
| Hispanic | 24 (31.2) | 82 (34.7) | |
| Others | 2 (2.6) | 7 (2.9) | |
| Family history of | |||
| Type 2 Diabetes | 21 (27.3) | 59 (25.0) | 0.556 |
| MetS (>2 components) | 16 (20.8) | 52 (22.0) | |
| Previous history of | |||
| - Gestational DM | 4 (5.2) | 9 (3.8) | 0.513 |
| - Miscarriages | 24 (31.2) | 87 (36.8) | |
| Educational status | |||
| Elementary education | 8 (10.4) | 16 (6.8) | 0.303 |
| Secondary School | 37 (48.1) | 103 (43.7) | |
| University Degree | 31 (40.3) | 115 (48.7) | |
| UNK | 1 (1.3) | 2 (0.8) | |
| Employed | 64 (83.1) | 181 (76.7) | 0.453 |
| Number of pregnancies | |||
| Primiparous | 29 (37.7) | 76 (32.3) | 0.760 |
| Second pregnancy | 28 (36.4) | 94 (40.0) | |
| >2 pregnancies | 20 (25.9) | 39 (22.5) | |
| Smoker | |||
| Never | 40 (51.9) | 132 (55.9) | 0.843 |
| Current | 7 (9.1) | 16 (6.8) | |
| Gestational Age (weeks) at baseline | 12.2 ± 0.7 | 12.1 ± 0.5 | 0.135 |
| Pre-pregnancy Body Weight (kg) | 62.0 (55.5–70.5) | 58.0 (54.0–65.0) | 0.017 |
| Pregnancy Body Weight at entry (kg) | 62.0 (56.6–72.5) | 60.0 (55.0–67.4) | 0.047 |
| Weight gain at 8–12 GW | 2.0 (0.0–3.0) | 2.0 (0.2–3.5) | 0.492 |
| Pre-pregnancy BMI (kg/m2) | 24.0 (20.8–27.4) | 22.2 (20.4–24.8) | 0.006 |
| Systolic BP/Diastolic BP (mm Hg) | 107 ± 11/66 ± 9 | 105 ± 9/63 ± 8 | 0.020/0.314 |
| Fasting Blood Glucose (mg/dL) | 81.9 ± 6.2 | 80.5 ± 6.3 | 0.084 |
| A1c (%) | 5.2 ± 0.2 | 5.0 ± 0.1 | 0.540 |
| Fasting Cholesterol (mg/dL) | 179 ± 35 | 174 ± 28 | 0.329 |
| Fasting Tryglycerides (mg/dL) | 96 ± 44 | 85 ± 50 | 0.139 |
| Fasting Plasma Insulin (µUI/mL) | 11.9 (6.8–24.7) | 10.8 (6.6–17.7) | 0.322 |
| HOMA-IR | 2.5 (1.3–5.0) | 2.3 (1.3–3.5) | 0.324 |
| Adiponectin (µg/mL) | 15.3 (11.3–24.9) | 20.0 (14.8–26.4) | 0.008 |
| IL-6 (pg/mL) | 3.4 (0.9–7.3) | 2.2 (0.1–5.7) | 0.092 |
| Leptin (ng/mL) | 8.8 (5.2–13.2) | 6.4 (3.6–11.6) | 0.099 |
| TNF-α (pg/mL) | 3.2 (2.0–4.7) | 2.4 (1.8–3.5) | 0.010 |
| TSH µUI/mL | 1.84 ± 1.21 | 1.94 ± 1.36 | 0.608 |
| FT4 (pg/mL) | 8.60 ± 1.39 | 8.76 ± 1.49 | 0.471 |
| MEDAS Score | 5.1 ± 1.6 | 5.0 ± 1.7 | 0.665 |
| Physical Activity Score | −1.9 ± 0.9 | −1.8 ± 1.1 | 0.605 |
| Nutrition Score | 0.9 ± 3.0 | 0.7 ± 3.3 | 0.517 |
| Intervention Group n (%) | 37 (48.1) | 135 (57.2) | 0.102 |
| Control Group | Intervention Group | |||||
|---|---|---|---|---|---|---|
| All | Glucose Regulation | All | Glucose Regulation | |||
| NGT | GDM | NGT | GDM | |||
| N | 141 | 101 | 40 | 172 | 135 | 37 |
| Pregestational | ||||||
| Nutrition Score | 0.9 ± 3.3 | 0.7 ± 3.3 | 1.3 ± 3.4 | 0.6 ± 3.2 | 0.6 ± 3.3 | 0.7 ± 2.6 |
| Phy_Activity_S | −1.7 ± 1.1 | −1.7 ± 1.1 | −1.8 ± 1.0 | −1.9 ± 1.0 | −1.9 ± 1.0 | −1.9 ± 0.9 |
| MEDAS Score | 4.8 ± 1.7 | 4.8 ± 1.7 | 4.8 ± 1.8 | 5.1 ± 1.6 | 5.1 ±1.7 | 5.3 ± 1.3 |
| 24–28 GW | ||||||
| Nutrition Score | 1.3 ± 3.5 | 1.0 ± 3.2 | 2.2 ± 3.9 | 4.7 ± 3.0 ***a | 4.8 ± 3.2 ***a | 4.2 ± 2.4 **a |
| Phy_Activity_S | −1.8 ± 1.0 | −1.7 ± 1.0 | −1.9 ± 1.0 | −1.9 ± 0.9 | −1.9 ± 0.9 | −1.9 ± 0.8 |
| MEDAS Score | 4.7 ± 1.7 | 4.5 ± 1.6 | 4.9 ± 2.0 | 6.7 ± 1.8 ***a | 6.7 ± 1.8 ***a | 6.5 ± 1.7 **a |
| 2–3 years PD | ||||||
| Nutrition Score | 2.8 ± 3.8 b | 2.2 ± 3.9 b | 3.8 ± 3.4 b | 3.4 ± 3.6 b | 3.2 ± 3.8 b | 3.9 ± 3.0 b |
| Phy_Activity_S | −1.6 ± 1.0 | −1.6 ± 1.1 | −1.7 ± 0.8 | −1.7 ± 0.9 | −1.7 ± 1.0 | −1.9 ± 0.9 |
| MEDAS Score | 6.1 ± 1.9 b | 5.9 ± 1.9 b | 6.5 ± 1.7 b | 6.6 ± 1.9 *a | 6.5 ± 1.9 *a | 6.9 ± 2.0 a |
| NGT (n = 236) | GDM (n = 77) | |||
|---|---|---|---|---|
| 24–28 GW | 2–3 Years PD | 24–28 GW | 2–3 Years PD | |
| Body Weight (Kg) | 67.4 ± 10.9 | 63.9 ± 12.9 | 70.1 ± 11.0 | 65.4 ± 12.8 |
| FP Glucose (mg/dL) | 83.5 ± 5.1 | 91.9 ± 6.1 | 92.6 ± 6.7 *** | 97.4 ± 7.1 *** |
| 1 h OGTT (mg/dL) | 118.8 ± 27.4 | n.a. | 159.4 ± 33.3 *** | n.a. |
| 2 h OGTT (mg/dL) | 104.5 ± 20.8 | 95.0 ± 19.4 | 135.1 ± 31.3 *** | 108.3 ± 33.4 ** |
| sBP (mm Hg) | 104 ± 11 | 111 ± 12 | 108 ± 11 * | 113 ± 10 |
| dBP (mm Hg) | 62 ± 9 | 71 ± 9 | 65 ± 8 * | 73 ± 8 * |
| T-Chol. (mg/dL) | 255 ± 44 | 176 ± 29 | 250 ± 46 | 183 ± 34 |
| Triglycerides (g/L) | 162 ± 55 | 75 ± 42 | 168 ± 45 | 92 ± 55 ** |
| HbA1c-IFCC % | 4.9 ± 0.3 | 5.3 ± 0.3 | 5.1 ± 0.3 *** | 5.5 ± 0.3 ** |
| TSH µUI/mL | 2.0 ± 1.0 | 1.9 ± 1.0 | 2.1 ± 1.0 | 1.9 ± 1.0 |
| FT4 (pg/mL) | 7.1 ± 1.1 | 8.3 ± 1.1 | 6.8 ± 1.0 | 8.1 ± 1.3 |
| FP insulin (µUI/mL) | 9.4 (7.0–12.8) | 8.7 (6.7–11.9) | 11.4 (8.6–16.5) *** | 10.5 (8.1–16.3) ** |
| Increase BS | 5.8 ± 15.4 | 6.1 ± 17.5 | 7.1 ± 7.0 * | 11.1 ± 16.9 * |
| Change (%) | 32 ± 8 | 28 ± 5 | 30 ± 3 | 35 ± 4 * |
| HOMA-IR | 1.8 (1.4–2.7) | 1.9 (1.5–2.8) | 2.3 (1.3–3.9) * | 2.5 (1.9–3.9) * |
| Increase BL | −0.7 ± 4.5 | 0.4 ± 5.2 | 0.1 ± 3.2 * | −1.2 ± 4.4 ** |
| Change (%) | 7 ± 4 | 7 ± 2 | 8 ± 4 | 20 ± 11 ** |
| Adiponectin (µg/mL) | 17 (13–22) | 16.9 (12.0–21.8) | 12.9 (9.8–17.2) *** | 13.7 (9.5–17.3) ** |
| Increase BL | −2.6 ± 7.1 | −3.5 ± 7.2 | −3.6 ± 6.8 | −3.5 ± 6.9 |
| Change (%) | 11 ± 4 | 13 ± 4 | 20 ± 3 | 21 ± 3 |
| IL-6 (pg/mL) | 2.1 (0.1–5.1) | 2.6 (0.1–6.2) | 2.8 (1.3–7.3) | 3.4 (1.0–10.5) * |
| Increase BL | 0.3 ± 7.0 | −0.1 ± 13.3 | 0.8 ± 4.5 | −0.5 ± 7.5 |
| Change (%) | 16 ± 48 | 53 ± 19 | 24. ± 21 | −42 ± 19 |
| Leptin (ng/mL) | 9.6 (6.0–15.1) | 6.4 (3.4–11.9) | 14.6 (9.2–19.4) * | 9.9 (5.6–15.3) ** |
| Increase BL | 3.6 ± 5.8 | 0.2 ± 5.1 | 5.6 ± 6.4 ** | 1.5 ± 7.3 * |
| Change (%) | 39 ± 9 | 2 ± 1 | 41 ± 7 | 25 ± 4 * |
| TNF-α (pg/mL) | 2.7 (1.8–3.9) | 2.8 (1.9–4.2) | 3.0 (2.0–4.4) | 3.6 (2.5–4.6) * |
| Increase BL | 0.3 ± 2.2 | 1.8 ± 1.9 | 1.9 ± 1.4 | 1.2 ± 1.6 |
| Change (%) | 10 ± 9.1 | 19 ± 8.5 | 38 ± 3 | 31 ± 5 |
| miR-222-3p | 1.20 (0.28–1.85) | 0.99 (0.21–1.70) | 1.24 (0.58–2.01) | 1.45 (0.76–2.21) * |
| miR-103a-3p | 0.92 (−0.90–2.24) | 1.44 (−0.21–2.53) | 1.18 (−0.25–1.91) | 1.58 (0.21–2.62) |
| miR-132 | 0.18 (−0.35–0.85) | 0.05 (−0.55–0.75) | 0.18 (−0.59–0.96 | 0.45 (−0.09–0.94) |
| miR-29a-3p | 0.26 (−0.95–1.67) | 0.11 (−1.07–1.54) | 0.21 (−0.52–2.06) | 0.38 (−0.69–1.81) |
| Contral Group (n = 141) | Intervention Group (n = 172) | |||
|---|---|---|---|---|
| 24–28 GW | 2–3 Years PD | 24–28 GW | 2–3 Years PD | |
| Body Weight (Kg) | 68.1 ± 11.8 | 64.4 ± 14.6 | 68.1 ± 10.3 | 64.2 ± 11.2 |
| FP Glucose (mg/dL) | 86.3 ± 6.7 | 93.6 ± 6.9 | 85.3 ± 6.8 * | 92.9 ± 6.7 |
| 1 h OGTT (mg/dL) | 126.8 ± 31.3 | n.a. | 124.2 ± 33.5 * | n.a. |
| 2 h OGTT (mg/dL) | 110.0 ± 27.0 | 95.0 ± 23.7 | 109.6 ± 24.5 | 99.3 ± 25.7 |
| sBP (mm Hg) | 104 ± 11 | 111 ± 12 | 105 ± 12 | 112 ± 12 |
| dBP (mm Hg) | 62 ± 9 | 71 ± 8 | 63 ± 9 | 71 ± 9 |
| T-Chol. (mg/dL) | 250 ± 47 | 174 ± 29 | 257 ± 42 | 180 ± 31 |
| Triglycerides (g/L) | 162 ± 52 | 83 ± 43 | 164 ± 54 | 76 ± 49 |
| HbA1c-IFCC % | 5.1 ± 0.3 | 5.3 ± 0.3 | 5.0 ± 0.3 * | 5.3 ± 0.3 |
| TSH µUI/mL | 1.9 ± 1.0 | 1.9 ± 1.1 | 2.1 ± 0.9 | 1.9 ± 0.9 |
| FT4 (pg/mL) | 7.2 ± 1.1 | 8.3 ± 1.1 | 7.0 ± 1.1 | 8.1 ± 1.3 |
| FP insulin (µUI/mL) | 10.8 (8.3–15.7) | 10.2 (7.7–14.2) | 9.0 (6.8–12.0) *** | 8.6 (6.7–11.6) ** |
| Increase BL | 6.5 ± 13.2 | 8.6 ± 20.2 | 5.9 ± 14.4 | 6.2 ± 14.9 |
| Change BL | 34 ± 6 | 34 ± 5 | 29 ± 7 | 26 ± 4 |
| HOMA-IR | 2.3 (1.7–3.3) | 2.3 (1.7–3.4) | 1.9 (1.4–2.6) * | 1.9 (1.5–2.8) * |
| Increase BL | 0.7 ± 4.7 | 0.1 ± 5.9 | 0.5 ± 3.9 * | −0.1 ± 4.2 * |
| Change BL (%) | 9 ± 7 | 5 ± 8 | 7 ± 3 | −6 ± 9 * |
| Adiponectin (µg/mL) | 17.0 (12.1–23.0) | 16.2 (11.2–22.6) | 15.5 (12.3–18.8) | 15.3 (11.2–18.9) |
| Increase BL | −2.9 ± 7.1 | −3.8 ± 7.8 | −2.5 ± 6.9 | −3.0 ± 6.1 |
| Change BL (%) | −10 ± 3 | −10 ± 4 | −10 ± 3 | −10 ± 3 |
| IL-6 (pg/mL) | 2.9 (1.9–7.1) | 3.0 (1.7–7.5) | 1.3 (0–4.8) *** | 1.6 (0.1–6.9) ** |
| Increase BL | 0.8 ± 5.7 | 0.3 ± 8.2 | −1.1 ± 7.1 | −0.8 ± 15.0 |
| Change BL (%) | 16 ± 48.4 | 36.8 ± 19.3 | 24 ± 21.4 | 65 ± 21.7 |
| Leptin (ng/mL) | 11.8 (7.6–19.5) | 7.5 (3.4–15.3) | 9.5 (6.3–14.7) ** | 7.4 (4.2–11.5) |
| Increase BL | 5.0 ± 6.6 | 0.5 ± 6.6 | 3.1 ± 5.2 ** | 0.6 ± 4.8 * |
| Change BL (%) | 9 ± 2 | 2 ± 1 | 8 ± 2 | 4 ± 10 |
| TNF-α (pg/mL) | 2.9 (1.9–4.5) | 3.3 (2.2–4.4) | 2.7 (1.8–3.6) | 2.8 (1.8–4.2) |
| Increase BL | 1.9 ± 2.5 | 1.1 ± 1.2 | 0.9 ± 1.9 | 0.8 ± 2.3 |
| Change BL (%) | −8 ± 9 | −10 ± 6 | 25 ± 2 | 27 ± 4 |
| miR-222-3p | 1.04 (−0.5–1.93) | 0.85 (−0.11–1.64) | 1.29 (0.71–1.87) *** | 1.11 (0.62–1.98) *** |
| miR-103a-3p | −0.12 (−1.48–1.20) | 0.35 (−0.92–1.80) | 1.57 (0.41–2.47) *** | 2.02 (0.52–2.80) *** |
| miR-132-3p | 0.25 (−0.2–0.89) | 0.34 (−0.1–0.81) | 0.17 (−0.51–0.83) | −0.03 (−0.58–0.86) |
| miR-29a-3p | 0.09 (−0.82–1.43) | 0.28 (−0.75–1.41) | 0.29 (−1.01–1.93) | 0.06 (−1.14–1.77) |
| NGT n = 236 | GDM n = 77 | |
|---|---|---|
| BMI (Kg/m2) | 24.2 ± 4.6 | 25.0 ± 4.7 * |
| WC (cm) | 81.4 ± 11.1 | 84.7 ± 12.8 * |
| Estimated Resting Energy (Kcal/day) | 1385 ± 176 | 1402 ± 225 |
| Total Energy (Kcal/day) | 2343 ± 325 | 2352 ± 276 |
| Fat Mass (kg) | 21.3 ± 9.1 | 22.7 ± 9.0 |
| Lean mass (kg) | 41.4 ± 6.0 | 41.9 ± 6.4 |
| Skeletal Muscle Mass (kg) | 18.9 ± 5.4 | 19.1 ± 3.4 |
| Body Water (L) | 31.2 ± 4.9 | 32.3 ± 5.9 |
| BMI >25 (Kg/m2) | 100 (42.5) | 44 (57.1) * |
| WC > 89.5 cm | 56 (23.7) | 32 (41.6) ** |
| Abnormal Glucose Regulation | 28 (10.6) | 25 (36.4) *** |
| IFG | 21 (8.9) | 24 (31.2) *** |
| IGT | 6 (2.5) | 10 (12.5) ** |
| Prediabetes (A1c > 5.7%) | 11(4.8) | 15 (19.7) *** |
| sBP > 130 mm Hg | 3 (1.2) | 0 |
| dBP > 85 mm Hg | 29 (12.4) | 11 (13.8) |
| TG > 150 mg/dL | 12 (5.1) | 9 (11.7) * |
| HDL < 45 mg/dL | 25 (10.6) | 8 (10.5) |
| Metabolic Syndrome | 26 (11.0) | 18 (23.4) * |
| Crude | Adjusted for ’Pre-Pregnancy BMI (kg/m2) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| miR-222-3p | miR-103a-3p | miR-222-3p | miR103a-3p | |||||||||||||
| 24–28 GW | 2–3 Yrs PD | 24–28 GW | 2–3 Yrs PD | 24–28 GW | 2–3 Yrs PD | 24–28 GW | 2–3 Yrs PD | |||||||||
| R | p-Value | R | p-Value | R | p-Value | R | p-Value | R | p-Value | R | p-Value | R | p-Value | R | p-Value | |
| Pre-pregnancy BMI (kg/m2) | 0.111 | 0.079 | 0.155 | 0.014 | 0.081 | 0.198 | 0.13 | 0.039 | ||||||||
| Insulin µUI/mL | ||||||||||||||||
| FP 24 GW | −0.127 | 0.044 | −0.108 | 0.091 | −0.165 | 0.008 | −0.148 | 0.018 | −0.157 | 0.013 | −0.152 | 0.018 | −0.189 | 0.003 | −0.184 | 0.003 |
| Change at 24 GW | 0.137 | 0.03 | 0.087 | 0.174 | 0.151 | 0.016 | 0.162 | 0.01 | 0.151 | 0.017 | 0.106 | 0.1 | 0.162 | 0.01 | 0.179 | 0.005 |
| Change at 2 yrs PD | 0.092 | 0.146 | 0.026 | 0.686 | 0.111 | 0.078 | 0.116 | 0.066 | 0.118 | 0.064 | 0.057 | 0.376 | 0.131 | 0.037 | 0.146 | 0.02 |
| HOMA-IR | ||||||||||||||||
| 24 GW | −0.119 | 0.06 | −0.098 | 0.125 | −0.152 | 0.015 | −0.133 | 0.035 | −0.153 | 0.016 | −0.147 | 0.022 | −0.179 | 0.004 | −0.173 | 0.006 |
| Change at 24 GW | 0.128 | 0.043 | 0.078 | 0.222 | 0.149 | 0.018 | 0.155 | 0.014 | 0.144 | 0.023 | 0.099 | 0.122 | 0.161 | 0.01 | 0.174 | 0.006 |
| Change at 2 yrs PD | 0.079 | 0.211 | 0.025 | 0.701 | 0.103 | 0.1 | 0.108 | 0.087 | 0.105 | 0.099 | 0.056 | 0.385 | 0.124 | 0.049 | 0.139 | 0.028 |
| Leptin (ng/mL) | ||||||||||||||||
| 12 GW | 0.156 | 0.025 | 0.193 | 0.006 | 0.022 | 0.75 | 0.093 | 0.18 | 0.08 | 0.25 | 0.093 | 0.188 | −0.074 | 0.283 | 0.001 | 0.989 |
| 24 GW | −0.028 | 0.693 | 0.024 | 0.732 | −0.149 | 0.03 | −0.12 | 0.083 | −0.117 | 0.093 | −0.089 | 0.209 | −0.247 | <0.001 | −0.226 | 0.001 |
| 2 yrs PD | 0.029 | 0.677 | 0.09 | 0.201 | −0.02 | 0.775 | 0.019 | 0.786 | −0.103 | 0.143 | −0.064 | 0.37 | −0.148 | 0.032 | −0.114 | 0.099 |
| Change at 24 GW | 0.23 | <0.001 | 0.239 | <0.001 | 0.202 | 0.003 | 0.269 | <0.001 | 0.2 | 0.004 | 0.199 | 0.005 | 0.176 | 0.011 | 0.242 | <0.001 |
| Change at 2 yrs PD | 0.154 | 0.026 | 0.146 | 0.038 | 0.032 | 0.646 | 0.098 | 0.155 | 0.18 | 0.01 | 0.177 | 0.012 | 0.049 | 0.477 | 0.121 | 0.081 |
| Adiponectin (µg/mL) | ||||||||||||||||
| 2 yrs PD | −0.123 | 0.104 | −0.163 | 0.032 | −0.034 | 0.649 | −0.064 | 0.394 | −0.093 | 0.221 | −0.122 | 0.112 | −0.001 | 0.989 | −0.022 | 0.772 |
| TNF-α (pg/mL) | ||||||||||||||||
| 12GW | −0.075 | 0.281 | 0.087 | 0.217 | −0.16 | 0.02 | −0.055 | 0.424 | −0.09 | 0.198 | 0.071 | 0.316 | −0.172 | 0.012 | −0.067 | 0.333 |
| 24GW | −0.14 | 0.044 | 0.021 | 0.762 | −0.204 | 0.003 | −0.102 | 0.14 | −0.153 | 0.028 | 0.005 | 0.949 | −0.215 | 0.002 | −0.111 | 0.108 |
| IL-6 (pg/mL) | ||||||||||||||||
| 12 GW | −0.152 | 0.028 | −0.008 | 0.911 | −0.243 | <0.001 | −0.147 | 0.033 | −0.151 | 0.031 | −0.006 | 0.935 | −0.242 | <0.001 | −0.145 | 0.037 |
| 24 GW | −0.151 | 0.029 | 0 | 0.995 | −0.307 | <0.001 | −0.156 | 0.023 | −0.165 | 0.018 | −0.016 | 0.826 | −0.318 | <0.001 | −0.168 | 0.015 |
| 2 yrs PD | −0.092 | 0.184 | −0.003 | 0.971 | −0.238 | <0.001 | −0.14 | 0.042 | −0.106 | 0.128 | −0.019 | 0.784 | −0.25 | <0.001 | −0.154 | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, J.; Barabash, A.; Garcia de la Torre, N.; De Miguel, P.; Melero, V.; del Valle, L.; Moraga, I.; Familiar, C.; Durán, A.; Torrejón, M.J.; et al. The Relationship between Serum Adipokines, miR-222-3p, miR-103a-3p and Glucose Regulation in Pregnancy and Two to Three Years Post-Delivery in Women with Gestational Diabetes Mellitus Adhering to Mediterranean Diet Recommendations. Nutrients 2022, 14, 4712. https://doi.org/10.3390/nu14224712
Valerio J, Barabash A, Garcia de la Torre N, De Miguel P, Melero V, del Valle L, Moraga I, Familiar C, Durán A, Torrejón MJ, et al. The Relationship between Serum Adipokines, miR-222-3p, miR-103a-3p and Glucose Regulation in Pregnancy and Two to Three Years Post-Delivery in Women with Gestational Diabetes Mellitus Adhering to Mediterranean Diet Recommendations. Nutrients. 2022; 14(22):4712. https://doi.org/10.3390/nu14224712
Chicago/Turabian StyleValerio, Johanna, Ana Barabash, Nuria Garcia de la Torre, Paz De Miguel, Verónica Melero, Laura del Valle, Inmaculada Moraga, Cristina Familiar, Alejandra Durán, Maria Jose Torrejón, and et al. 2022. "The Relationship between Serum Adipokines, miR-222-3p, miR-103a-3p and Glucose Regulation in Pregnancy and Two to Three Years Post-Delivery in Women with Gestational Diabetes Mellitus Adhering to Mediterranean Diet Recommendations" Nutrients 14, no. 22: 4712. https://doi.org/10.3390/nu14224712