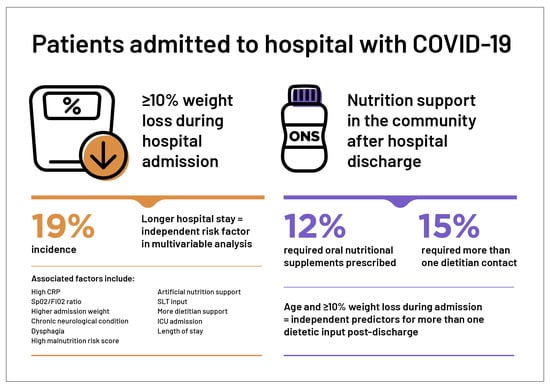
Factors Associated with Significant Weight Loss in Hospitalised Patients with COVID-19: A Retrospective Cohort Study in a Large Teaching Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.1.1. Ethical Statement
2.1.2. Study Population
2.1.3. Data Collection
2.1.4. Primary Outcome
2.1.5. Secondary Outcomes
2.1.6. Demographics
2.1.7. Anthropometry
2.1.8. Malnutrition Risk on Admission
2.1.9. Disease-Related Factors
2.1.10. Dietary Management
2.1.11. Post-Discharge
2.1.12. Statistical Analysis
3. Results
3.1. Primary Outcome
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aeberhard, C.; Birrenbach, T.; Joray, M.; Muhlebach, S.; Perrig, M.; Stanga, Z. Simple training tool is insufficient for appropriate diagnosis and treatment of malnutrition: A pre-post intervention study in a tertiary center. Nutrition 2016, 32, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Lechtenboehmer, C.; Bally, M.; Fehr, R.; Deiss, M.; Faessler, L.; Kutz, A.; Steiner, D.; Rast, A.C.; Laukemann, S.; et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition 2015, 31, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Schuetz, P.; Bounoure, L.; Austin, P.; Ballesteros-Pomar, M.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; Poulia, K.A.; et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin. Nutr. 2018, 37, 336–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubrak, C.; Jensen, L. Malnutrition in acute care patients: A narrative review. Int. J. Nurs. Stud. 2007, 44, 1036–1054. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Marques-Vidal, P. The economic cost of hospital malnutrition in Europe; a narrative review. Clin. Nutr. ESPEN 2015, 10, e89–e94. [Google Scholar] [CrossRef] [Green Version]
- Schuetz, P. “Eat your lunch!”—Controversies in the nutrition of the acutely, non-critically ill medical inpatient. Swiss Med. Wkly. 2015, 145, w14132. [Google Scholar] [CrossRef] [Green Version]
- Schuetz, P. Food for thought: Why does the medical community struggle with research about nutritional therapy in the acute care setting? BMC Med. 2017, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Tao, Z.W.; Wang, L.; Yuan, M.L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.G.; et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. (Engl.) 2020, 133, 1032–1038. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Haraj, N.E.; El Aziz, S.; Chadli, A.; Dafir, A.; Mjabber, A.; Aissaoui, O.; Barrou, L.; El Kettani El Hamidi, C.; Nsiri, A.; Al Harrar, R.; et al. Nutritional status assessment in patients with Covid-19 after discharge from the intensive care unit. Clin. Nutr. ESPEN 2021, 41, 423–428. [Google Scholar] [CrossRef]
- Coronaviridae Study Group of the International Committee on Taxonomy of V. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Abate, S.M.; Chekole, Y.A.; Estifanos, M.B.; Abate, K.H.; Kabthymer, R.H. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 43, 174–183. [Google Scholar] [CrossRef]
- Assennato, S.M.; Ritchie, A.V.; Nadala, C.; Goel, N.; Tie, C.; Nadala, L.M.; Zhang, H.; Datir, R.; Gupta, R.K.; Curran, M.D.; et al. Performance Evaluation of the SAMBA II SARS-CoV-2 Test for Point-of-Care Detection of SARS-CoV-2. J. Clin. Microbiol. 2020, 59, e01262-20. [Google Scholar] [CrossRef]
- Iacobucci, G. COVID-19: What is the UK’s testing strategy? BMJ 2020, 368, m1222. [Google Scholar] [CrossRef] [Green Version]
- Bramer, G.R. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat. Q 1988, 41, 32–36. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition; NICE Clinical Guidelines, No. 32; National Institute for Health and Care Excellence (NICE): London, UK, 2017; ISBN 13:78-1-4731-1910-9. [Google Scholar]
- World Health Organization. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- Reilly, H.M.; Martineau, J.K.; Moran, A.; Kennedy, H. Nutritional screening--evaluation and implementation of a simple Nutrition Risk Score. Clin. Nutr. 1995, 14, 269–273. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with High Risk for Severe COVID-19: Information for Healthcare Providers. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 5 April 2021).
- Centers for Disease Control and Prevention. Science Brief: Evidence Used to Update the List of Underlying Medical Conditions that Increase a Person’s Risk of Severe Illness from COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlying-evidence-table.html (accessed on 5 April 2021).
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef]
- De Lorenzo, R.; Conte, C.; Lanzani, C.; Benedetti, F.; Roveri, L.; Mazza, M.G.; Brioni, E.; Giacalone, G.; Canti, V.; Sofia, V.; et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS ONE 2020, 15, e0239570. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; D’Amico, M.; Sofia, V.; Roveri, L.; Mele, R.; Saibene, A.; Rovere-Querini, P.; Conte, C. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin. Nutr. 2021, 40, 2420–2426. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; Mao, Z.; Wang, W.; Hu, H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur. J. Clin. Nutr. 2020, 74, 876–883. [Google Scholar] [CrossRef]
- Bedock, D.; Bel Lassen, P.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef]
- Fiorindi, C.; Campani, F.; Rasero, L.; Campani, C.; Livi, L.; Giovannoni, L.; Amato, C.; Giudici, F.; Bartoloni, A.; Fattirolli, F.; et al. Prevalence of nutritional risk and malnutrition during and after hospitalization for COVID-19 infection: Preliminary results of a single-centre experience. Clin. Nutr. ESPEN 2021, 45, 351–355. [Google Scholar] [CrossRef]
- Yu, Y.; Ye, J.; Chen, M.; Jiang, C.; Lin, W.; Lu, Y.; Ye, H.; Li, Y.; Wang, Y.; Liao, Q.; et al. Malnutrition Prolongs the Hospitalization of Patients with COVID-19 Infection: A Clinical Epidemiological Analysis. J. Nutr. Health Aging 2021, 25, 369–373. [Google Scholar] [CrossRef]
- Herridge, M.S.; Cheung, A.M.; Tansey, C.M.; Matte-Martyn, A.; Diaz-Granados, N.; Al-Saidi, F.; Cooper, A.B.; Guest, C.B.; Mazer, C.D.; Mehta, S.; et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003, 348, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Camprubi-Robles, M.; Bear, D.E.; Cederholm, T.; Malafarina, V.; Welch, A.A.; Cruz-Jentoft, A.J. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef] [Green Version]
- Cawood, A.L.; Walters, E.R.; Smith, T.R.; Sipaul, R.H.; Stratton, R.J. A Review of Nutrition Support Guidelines for Individuals with or Recovering from COVID-19 in the Community. Nutrients 2020, 12, 3230. [Google Scholar] [CrossRef]
- Breton, I. Nutritional and Metabolic Consequences of Neurological Diseases; Nutritional Support in Neurological Diseases, Topic 25, Module 25.1; The European Society for Clinical Nutrition and Metabolism: Madrid, Spain, 2016. [Google Scholar]
- Garcia-Azorin, D.; Martinez-Pias, E.; Trigo, J.; Hernandez-Perez, I.; Valle-Penacoba, G.; Talavera, B.; Simon-Campo, P.; de Lera, M.; Chavarria-Miranda, A.; Lopez-Sanz, C.; et al. Neurological Comorbidity Is a Predictor of Death in COVID-19 Disease: A Cohort Study on 576 Patients. Front. Neurol. 2020, 11, 781. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Hughes, R.A.; Heron, J.; Sterne, J.A.C.; Tilling, K. Accounting for missing data in statistical analyses: Multiple imputation is not always the answer. Int. J. Epidemiol. 2019, 48, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. Endorsed by the E.C. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total Sample (n = 288) | Weight Loss <10% (n = 234) | Weight Loss ≥10% (n = 54) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 72.0 (59.0–82.0) | 72.0 (61.0–82.0) | 69.5 (56.0–79.2) | 0.319 |
| Sex, % (n.) | 0.144 | |||
| Male | 59.7 (172) | 57.7 (135) | 68.5 (37) | |
| Female | 40.3 (116) | 42.3 (99) | 31.5 (17) | |
| Ethnicity, % (n.) | 0.554 | |||
| White | 75.3 (217) | 76.1 (178) | 72.2 (39) | |
| Other | 24.7 (71) | 22.2 (52) | 27.8 (15) | |
| Comorbidities | ||||
| Chronic neurological conditions, % (n.) | 29.9 (86) | 26.9 (63) | 42.6 (23) | 0.023 |
| T2DM, % (n.) | 26.0 (75) | 25.6 (60) | 27.8 (15) | 0.095 |
| Hypertension, % (n.) | 52.8 (152) | 53.8 (126) | 48.1 (26) | 0.450 |
| Admission data | ||||
| GCS on admission | 15 (14–15) | 15 (14–15) | 14 (5–15) | 0.001 |
| NEWS2 on admission | 5 (2–7) | 4 (2–6) | 5 (2–8) | 0.149 |
| SpO2/FiO2 ratio on admission | 447.6 (323.2–461.9) | 450.0 (342.8–462.0) | 442.8 (285.4–457.1) | 0.081 |
| SpO2 on admission, % | 96 (94–97) | 96 (94–97) | 95 (93–97) | 0.687 |
| Respiratory rate on admission (breaths/min) | 20 (18–26) | 20 (18–26) | 20 (17–24) | 0.257 |
| CRP on admission, mg/dL | 76.0 (29.0–175.0) | 76.0 (25.0–177.0) | 81.0 (38.5–172.5) | 0.406 |
| During admission data | ||||
| CRP max, mg/dL | 191.0 (90.2–280.5) | 180.5 (79–286) | 228.5 (158–305) | 0.018 |
| CRP ≥ 178 mg/dl, % (n.) | 53.5 (153) | 50.0 (117) | 66.7 (36) | 0.031 |
| Oxygen therapy, % (n.) | ||||
| Invasive mechanical ventilation | 26.0 (75) | 21.8 (51) | 44.4 (24) | 0.001 |
| NIV | 15.3 (44) | 12.4 (29) | 27.8 (15) | 0.005 |
| CPAP | 14.2 (41) | 11.5 (27) | 25.9 (14) | 0.006 |
| Prone positioning | 12.8 (37) | 11.5 (27) | 18.5 (10) | 0.167 |
| HFNO | 7.6 (22) | 7.3 (17) | 9.3 (5) | 0.619 |
| RRT, % (n.) | 10.8 (31) | 7.7 (18) | 24.1 (13) | <0.001 |
| Length of hospital stay (days) | 16.9 (10.9–30.9) | 15.5 (6.4–24.0) | 35.8 (22.7–57.5) | <0.001 |
| Admitted to ICU, % (n.) | 33.3 (96) | 28.2 (66) | 55.6 (30) | <0.001 |
| Length of ICU stay (days) | 14.9 (3.1–24.9) | 12.6 (4.9–21.0) | 17.9 (7.7–32.0) | 0.068 |
| Deceased, % (n.) | 19.4 (56) | 21.4 (50) | 11.1 (6) | 0.086 |
| Readmitted within 30 days, % (n.) | 18.4 (53) | 18.8 (44) | 16.7 (9) | 0.715 |
| Variables | Total Sample (n = 288) | Weight Loss <10% (n = 234) | Weight Loss ≥ 10% (n = 54) | p Value |
|---|---|---|---|---|
| Admission data | ||||
| Weight (kg) | 75.3 (62.9–90) | 73.8 (62.3–88.2) | 80.1 (65.5–98.1) | 0.014 |
| BMI category, % (n.) | 0.122 | |||
| Underweight, BMI < 18.5 kg/m2 | 5.6 (16) | 6.4 (15) | 1.9 (1) | |
| Normal weight, BMI 18.5–24.9 kg/m2 | 37.5 (108) | 39.3 (92) | 29.6 (16) | |
| Overweight, BMI 25–29.9 kg/m2 | 27.8 (80) | 27.8 (65) | 27.8 (15) | |
| Obese, BMI ≥ 30 kg/m2 | 29.2 (84) | 26.5 (62) | 40.7 (22) | |
| Malnutrition screening tool score ≥ 6, % (n.) | 26.7 (77) | 23.5 (55) | 40.7 (22) | 0.012 |
| During admission data | ||||
| Taste changes/loss, % (n.) | 14.9 (43) | 13.2 (31) | 22.2 (12) | 0.095 |
| Smell changes/loss, % (n.) | 7.6 (22) | 5.6 (13) | 16.7 (9) | 0.006 |
| Dysphagia, % (n.) | 29.2 (84) | 25.2 (59) | 46.3 (25) | 0.002 |
| Anorexia/loss of appetite, % (n.) | 64.9 (187) | 64.1 (150) | 68.5 (37) | 0.540 |
| Seen by dietitian, % (n.) | 81.6 (235) | 78.6 (184) | 94.4 (51) | 0.007 |
| Number of total dietetic inputs | 5 (2–9) | 5 (2–8) | 8 (4–19) | <0.001 |
| Artificial feeding (EN, PN), % (n.) | 32.6 (94) | 28.6 (67) | 53.7 (29) | <0.001 |
| Duration of EN (days) | 25.0 (12.0–37.5) | 17.0 (10.7–30.0) | 36.0 (30.0–63.0) | <0.001 |
| Duration of PN in days | 5.0 (2.0–12.5) | 5.00 (2.0–12.0) | 5.00 (2.0–28.0) | 0.817 |
| ONS provided, % (n.) | 64.9 (187) | 62.8 (147) | 74.1 (40) | 0.118 |
| SLT assessment, % (n.) | 30.6 (88) | 24.4 (57) | 57.4 (31) | <0.001 |
| Variables | Post-Discharge Sample (n = 232) |
|---|---|
| Feeding route on discharge, % (n.) | |
| Oral | 94.4 (219) |
| AF | 8.6 (20) |
| Discharge destination, % (n.) | |
| Usual place of residence | 74.1 (172) |
| Private/NHS nursing/Residential home | 13.3 (31) |
| NHS hospital | 8.6 (20) |
| Other | 3.9 (9) |
| Received a dietetic call post-discharge, % (n.) | 50.4 (117) |
| Duration between discharge and dietetic call (days) | 9.0 (5.7–13.2) |
| More than one dietetic input after discharge, % (n) | 15.1 (35) |
| Referred to community or HEF dietitians, % (n.) | 13.4 (31) |
| ONS GP prescription requested, % (n.) | 11.6 (27) |
| Weight post-discharge (kg) | 72.4 (61.8–83.1) |
| Taste changes/loss, % (n.) | 7.8 (18) |
| Smell changes/loss, % (n.) | 3.4 (8) |
| Dysphagia, % (n.) | 11.2 (26) |
| Anorexia/loss of appetite, % (n.) | 15.1 (35) |
| ≥10% Weight Loss during Admission | ||
|---|---|---|
| Variables | OR (95% CI) | p Value |
| Age (years) | 1.02 (0.99–1.04) | 0.233 |
| Sex (male) | 1.32 (0.63–2.74) | 0.462 |
| Length of hospital stay (weeks) | 1.22 (1.08–1.38) | 0.001 |
| ICU admission | 1.51 (0.59–3.87) | 0.389 |
| CRP ≥178 mg/dl during admission | 1.15 (0.55–2.40) | 0.717 |
| Weight on admission (kg) | 1.04 (0.96–1.14) | 0.323 |
| Dysphagia on/during admission | 0.98 (0.44–2.13) | 0.931 |
| SpO2/FiO2 ratio on admission | 0.99 (0.99–1.00) | 0.553 |
| Chronic neurological conditions | 1.02 (0.48–2.19) | 0.959 |
| Malnutrition screening tool score ≥6 | 1.46 (0.66–3.24) | 0.346 |
| Required More Than One Dietetic Input Post-Discharge | ||
|---|---|---|
| Variables | OR (95% CI) | p Value |
| Age (years) | 1.03 (1.00–1.05) | 0.024 |
| Sex (male) | 1.45 (0.73–2.85) | 0.287 |
| CRP ≥178 mg/dl during admission | 1.51 (0.77–2.97) | 0.227 |
| ≥10% Weight loss during admission | 2.11 (1.03–4.34) | 0.041 |
| Artificial feeding during admission | 1.49 (0.71–3.12) | 0.290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannidi, D.; Patel, P.S.; Leventea, E.; Paciepnik, J.; Dobson, F.; Heyes, C.; Goudie, R.J.B.; Griep, L.M.O.; Preller, J.; Spillman, L.N. Factors Associated with Significant Weight Loss in Hospitalised Patients with COVID-19: A Retrospective Cohort Study in a Large Teaching Hospital. Nutrients 2022, 14, 4195. https://doi.org/10.3390/nu14194195
Zannidi D, Patel PS, Leventea E, Paciepnik J, Dobson F, Heyes C, Goudie RJB, Griep LMO, Preller J, Spillman LN. Factors Associated with Significant Weight Loss in Hospitalised Patients with COVID-19: A Retrospective Cohort Study in a Large Teaching Hospital. Nutrients. 2022; 14(19):4195. https://doi.org/10.3390/nu14194195
Chicago/Turabian StyleZannidi, Dimitra, Pinal S. Patel, Eleni Leventea, Jessica Paciepnik, Frances Dobson, Caroline Heyes, Robert J. B. Goudie, Linda M. Oude Griep, Jacobus Preller, and Lynsey N. Spillman. 2022. "Factors Associated with Significant Weight Loss in Hospitalised Patients with COVID-19: A Retrospective Cohort Study in a Large Teaching Hospital" Nutrients 14, no. 19: 4195. https://doi.org/10.3390/nu14194195