Prevalence of Zinc Deficiency in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Abstract
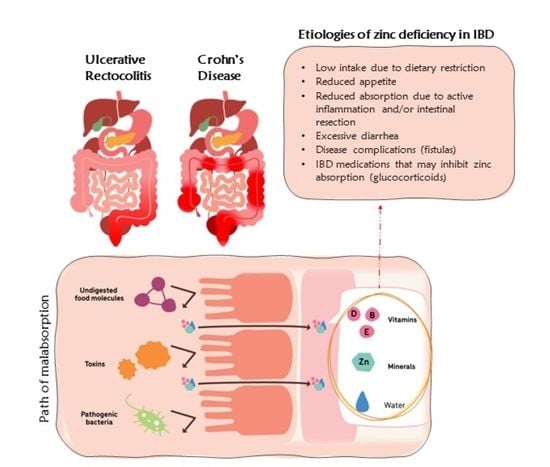
:1. Introduction
2. Methods
2.1. Search Strategy, Selection Criteria, and Data Extraction
2.2. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hess, S.Y.; Peerson, J.M.; King, J.C.; Brown, K.H. Use of Serum Zinc Concentration as an Indicator of Population Zinc Status. Food Nutr. Bull. 2007, 28, S403–S429. [Google Scholar] [CrossRef] [PubMed]
- Zinc. Available online: https://www.hsph.harvard.edu/nutritionsource/zinc/ (accessed on 10 May 2022).
- Institute of Medicine; Food and Nutrition Board; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Subcommittee of Interpretation and Uses of Dietary Reference Intakes; Subcommittee on Upper Reference Levels of Nutrients. Panel on Micronutrients Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2002; ISBN 9780309072908. [Google Scholar]
- US Department of Agriculture; A.R.S. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 10 May 2022).
- Sandström, B. Bioavailability of Zinc. Eur. J. Clin. Nutr. 1997, 51 (Suppl. 1), S17–S19. [Google Scholar] [PubMed]
- Wise, A. Phytate and Zinc Bioavailability. Int. J. Food Sci. Nutr. 1995, 46, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Iwaya, H.; Kashiwaya, M.; Shinoki, A.; Lee, J.-S.; Hayashi, K.; Hara, H.; Ishizuka, S. Marginal Zinc Deficiency Exacerbates Experimental Colitis Induced by Dextran Sulfate Sodium in Rats. J. Nutr. 2011, 141, 1077–1082. [Google Scholar] [CrossRef]
- Sturniolo, G.C.; Di Leo, V.; Ferronato, A.; D’Odorico, A.; D’Incà, R. Zinc Supplementation Tightens “Leaky Gut” in Crohn’s Disease. Inflamm. Bowel Dis. 2001, 7, 94–98. [Google Scholar] [CrossRef]
- Wong, C.P.; Rinaldi, N.A.; Ho, E. Zinc Deficiency Enhanced Inflammatory Response by Increasing Immune Cell Activation and Inducing IL6 Promoter Demethylation. Mol. Nutr. Food Res. 2015, 59, 991–999. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Belur, J.; Tompson, L.; Thornton, A.; Simon, M. Interrater Reliability in Systematic Review Methodology: Exploring Variation in Coder Decision-Making. Sociol. Methods Res. 2021, 50, 837–865. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing Risk of Bias in Prevalence Studies: Modification of an Existing Tool and Evidence of Interrater Agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Mark, A.G.; Rinawi, F.; Shamir, R.; Assa, A. Micronutrient Deficiencies in Children with Inflammatory Bowel Diseases. Nutr. Clin. Pract. 2020, 35, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Yoon, H.; Lim, S.; Sung, M.-K.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H.; Kim, J.S. Risk Factors for Vitamin D, Zinc, and Selenium Deficiencies in Korean Patients with Inflammatory Bowel Disease. Gut Liver 2017, 11, 363–369. [Google Scholar] [CrossRef] [PubMed]
- MacMaster, M.J.; Damianopoulou, S.; Thomson, C.; Talwar, D.; Stefanowicz, F.; Catchpole, A.; Gerasimidis, K.; Gaya, D.R. A Prospective Analysis of Micronutrient Status in Quiescent Inflammatory Bowel Disease. Clin. Nutr. 2021, 40, 327–331. [Google Scholar] [CrossRef]
- Schneider, T.; Caviezel, D.; Ayata, C.K.; Kiss, C.; Niess, J.H.; Hruz, P. The Copper/Zinc Ratio Correlates with Markers of Disease Activity in Patients with Inflammatory Bowel Disease. Crohns Colitis 360 2020, 2, otaa001. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency Is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, J.; Arai, K.; Kudo, T.; Nambu, R.; Tajiri, H.; Aomatsu, T.; Abe, N.; Kakiuchi, T.; Hashimoto, K.; Sogo, T.; et al. Serum Zinc and Selenium in Children with Inflammatory Bowel Disease: A Multicenter Study in Japan. Dig. Dis. Sci. 2021, 67, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Furukawa, S.; Katsurada, T.; Otagiri, S.; Yamanashi, K.; Nagashima, K.; Onishi, R.; Yagisawa, K.; Nishimura, H.; Ito, T.; et al. Effectiveness of Administering Zinc Acetate Hydrate to Patients with Inflammatory Bowel Disease and Zinc Deficiency: A Retrospective Observational Two-Center Study. Intestig. Res. 2022, 20, 78–89. [Google Scholar] [CrossRef]
- Soltani, Z.; Rafiei, F.; EBRAHIMi, A.; Rafiei, R. The Prevalence of Zinc Deficiency in Crohn’s Disease Patients. Maedica 2021, 16, 29–33. [Google Scholar]
- Cho, J.M.; Yang, H.R. Hair Mineral and Trace Element Contents as Reliable Markers of Nutritional Status Compared to Serum Levels of These Elements in Children Newly Diagnosed with Inflammatory Bowel Disease. Biol. Trace Elem. Res. 2018, 185, 20–29. [Google Scholar] [CrossRef]
- Prasad, A.S. Effects of Zinc Deficiency on Th1 and Th2 Cytokine Shifts. J. Infect. Dis. 2000, 182 (Suppl. 1), S62–S68. [Google Scholar] [CrossRef] [PubMed]
- Ranaldi, G.; Ferruzza, S.; Canali, R.; Leoni, G.; Zalewski, P.D.; Sambuy, Y.; Perozzi, G.; Murgia, C. Intracellular Zinc Is Required for Intestinal Cell Survival Signals Triggered by the Inflammatory Cytokine TNFα. J. Nutr. Biochem. 2013, 24, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected Vitamins and Trace Elements Support Immune Function by Strengthening Epithelial Barriers and Cellular and Humoral Immune Responses. Br. J. Nutr. 2007, 98 (Suppl. 1), S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc Absorption in Human Small Intestine. Am. J. Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef]
- Scarpellini, E.; Balsiger, L.M.; Maurizi, V.; Rinninella, E.; Gasbarrini, A.; Giostra, N.; Santori, P.; Abenavoli, L.; Rasetti, C. Zinc and Gut Microbiota in Health and Gastrointestinal Disease under the COVID-19 Suggestion. Biofactors 2022, 48, 294–306. [Google Scholar] [CrossRef]
- Sandstead, H.H. Zinc Deficiency. A Public Health Problem? Am. J. Dis. Child. 1991, 145, 853–859. [Google Scholar] [CrossRef]
- Shay, N.F.; Mangian, H.F. Neurobiology of Zinc-Influenced Eating Behavior. J. Nutr. 2000, 130, 1493S–1499S. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Bortone, I.; Griseta, C.; Sardone, R.; Lampignano, L.; Lozupone, M.; Solfrizzi, V.; Castellana, M.; Giannelli, G.; et al. Nutritional Domains in Frailty Tools: Working towards an Operational Definition of Nutritional Frailty. Ageing Res. Rev. 2020, 64, 101148. [Google Scholar] [CrossRef]
- Poursadegh, F.; Ahadi, M.; Vosoughinia, H.; Salehi, M.; Beheshti Namdar, A.; Farzanehfar, M.R.; Memar, B.; Ziaolhagh, R. A STROBE Compliant Observational Study on Trace Elements in Patients with Ulcerative Colitis and Their Relationship with Disease Activity. Medicine 2018, 97, e13523. [Google Scholar] [CrossRef]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN Micronutrient Guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]


| Authors, Year [Ref.] | Survey Year | Country | Study Design | Age (Years) | Sample Size | Sex (Female) | Serum Zinc Levels | Deficiency Cut-Off | Summary of Findings | Overall Risk of Bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD | UC | CD | UC | CD | UC | CD + UC | ||||||||
| Ehrlich, Shay, et al., 2020 [15] | 2000–2016 | Asia (Israel) | Longitudinal, 7-year | 14.1 (12–16) * | 13.5 (10.8–15.7) * | 225 | 38 | 96/225 (43%) | 18/38 (46%) | 70.5 ± 16.3 mcg/dL | ≤70 mcg/dL | The prevalence of zinc deficiency in patients with CD at diagnosis was 88% (CD) and 31.6% (UC) in patients with IBD. | Low risk | |
| CD + UC | CD | UC | CD + UC | CD+ UC | ||||||||||
| Han, Yoo Min, et al., 2017 [16] | 2013–2015 | Asia (Korea) | Cross-sectional | 32 (16–70) ♯ | 34 | 49 | 19/83 (22.9%) | 76.6 ± 14.9 mcg/dL | ≤70 mcg/dL | Many Korean patients with IBD have zinc deficiencies, suggesting the need to monitor levels of these micronutrients. | Moderate risk | |||
| CD | UC | CD | UC | CD | UC | CD | UC | |||||||
| Macmaster, Damianopoulou, et al., 2021 [17] | 2017–2018 | Europe (UK) | Cross-sectional | 48.0 (19.5–78.4) ♯ | 47.2 (21.0–78.5) ♯ | 59 | 30 | 37 (63%) | 16 (53%) | NA | Laboratory range (not specified) | Zinc deficiencies had been found in 23.7% (CD) and 76.6% (UC) of subjects with IBD | Moderate risk | |
| CD | UC | CD | UC | CD | UC | |||||||||
| Schneider, Caviezel, et al., 2020 [18] | 2016–2017 | Europe (Switzerland) | Cross-sectional | 41.32 (14.5) ‡ | 41.6 (13.7) ‡ | 98 | 56 | 48 (49%) | 31 (55%) | NA | <10.7 µmol/L | In this study, insufficient serum zinc concentrations were observed in 11.2% of patients with CD and in 14.3% of patients with UC | Moderate risk | |
| CD | UC | CD | UC | CD | UC | CD | UC | |||||||
| Siva, Rubin, et al., 2017 [19] | 2000–2015 | America (USA) | Longitudinal, 3-year | NA | NA | 773 | 223 | 421/773 (54%) | 107/223 (48%) | NA | <0.66 mg/mL | Patients with IBD with serum zinc deficiency are more likely to have adverse disease-specific outcomes | Low risk | |
| CD | UC | CD | UC | CD | UC | CD | UC | |||||||
| Ishihara, Arai, et al., 2021 [20] | 2018 | Asia (Japan) | Cross-sectional | 13 (4–16) ♯ | 11 (1–16) ♯ | 98 | 118 | 30/98 (31%) | 53/118 (45%) | 64 (33–124) μg/dL * | 69 (41–177) μg/dL * | <70 μg/dL | Prevalence of zinc deficiency in pediatric patients with IBD was 60.2% (CD) and 37.3% (UC) | Low risk |
| CD | UC | CD | UC | CD | UC | CD | UC | |||||||
| Sakurai, Furukawa, et al., 2022 [21] | 2017–2020 | Asia (Japan) | Longitudinal, 20 weeks | 39.5 (23–63) ♯ | 56.0 (28–87) ♯ | 276 | 206 | NA | 57.5 (31–74) μg/dL | 63 (46–74) μg/dL | <80 μg/dL | Zinc deficiencies had been found in 86.2% (CD) and 50.7% (UC) of IBD subjects | Moderate risk | |
| CD | CD | CD | CD | |||||||||||
| Soltani, Zahra, et al., 2021 [22] | 2018–2019 | Asia (Iran) | Cross-sectional | 39.2 ± 13.4 | 42 ± 16.2 | 65 | 49 (75.4%) | 86.2 ± 17.0 ng/dL | Laboratory range (not specified) | Zinc deficiency was observed in 21.5% of a CD sample | Moderate risk | |||
| CD | UC | CD | UC | CD | UC | CD | UC | |||||||
| Cho And Yang, 2018 [23] | 2012–2016 | Asia (Korea) | Cross-sectional | 14.4 (5.0–17.4) ♯ | 14.2 (9.9–17.4) ♯ | 49 | 16 | 16/49 (33%) | 9/16 (56%) | 71.5 (32.0–105.0) μg/dL ♯ | 77.0 (55.0–106.0) μg/dL ♯ | <70 μg/dL | Zinc deficiencywas found in 44.9% (CD) and 31.2% (UC) of IBD sample | Low risk |
| n | Authors, Year | Disease | Deficiency Cases | Total Cases | Prevalence (%) | CI 95% |
|---|---|---|---|---|---|---|
| 1 | Ehrlich, Shay, et al., 2020 [15] | CD | 198 | 225 | 88.00 | 0.83 to 0.92 |
| UC | 12 | 38 | 31.58 | 0.18 to 0.49 | ||
| 2 | Han, Yoo Min, et al., 2017 [16] | CD | 19 | 34 | 55.88 | 0.56 to 0.73 |
| UC | 13 | 49 | 26.53 | 0.15 to 0.41 | ||
| 3 | MacMaster, Damianopoulou, et al., 2021 [17] | CD | 14 | 59 | 23.73 | 0.14 to 0.37 |
| UC | 23 | 30 | 76.67 | 0.58 to 0.90 | ||
| 4 | Schneider, Caviezel, et al., 2020 [18] | CD | 11 | 98 | 11.22 | 0.06 to 0.19 |
| UC | 8 | 56 | 14.29 | 0.06 to 0.26 | ||
| 5 | Siva, Rubin, et al., 2017 [19] | CD | 326 | 773 | 42.17 | 0.39 to 0.46 |
| UC | 86 | 223 | 38.57 | 0.32 to 0.45 | ||
| 6 | Ishihara, Arai, et al., 2021 [20] | CD | 59 | 98 | 60.20 | 0.50 to 0.70 |
| UC | 44 | 118 | 37.29 | 0.29 to 0.47 | ||
| 7 | Sakurai, Furukawa, et al., 2022 [21] | CD | 238 | 276 | 86.23 | 0.82 to 0.90 |
| UC | 140 | 276 | 50.72 | 0.45 to 0.57 | ||
| 8 | Soltani, Zahra, et al., 2021 [22] | CD | 14 | 65 | 21.54 | 0.12 to 0.33 |
| 9 | Cho and Yang, 2018 [23] | CD | 22 | 49 | 44.90 | 0.31 to 0.60 |
| UC | 5 | 16 | 31.25 | 0.11 to 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupo, R.; Sila, A.; Castellana, F.; Bringiotti, R.; Curlo, M.; De Pergola, G.; De Nucci, S.; Giannelli, G.; Mastronardi, M.; Sardone, R. Prevalence of Zinc Deficiency in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4052. https://doi.org/10.3390/nu14194052
Zupo R, Sila A, Castellana F, Bringiotti R, Curlo M, De Pergola G, De Nucci S, Giannelli G, Mastronardi M, Sardone R. Prevalence of Zinc Deficiency in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(19):4052. https://doi.org/10.3390/nu14194052
Chicago/Turabian StyleZupo, Roberta, Annamaria Sila, Fabio Castellana, Roberto Bringiotti, Margherita Curlo, Giovanni De Pergola, Sara De Nucci, Gianluigi Giannelli, Mauro Mastronardi, and Rodolfo Sardone. 2022. "Prevalence of Zinc Deficiency in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis" Nutrients 14, no. 19: 4052. https://doi.org/10.3390/nu14194052