The Mutual Relationship among Cardiovascular Diseases and COVID-19: Focus on Micronutrients Imbalance
Abstract
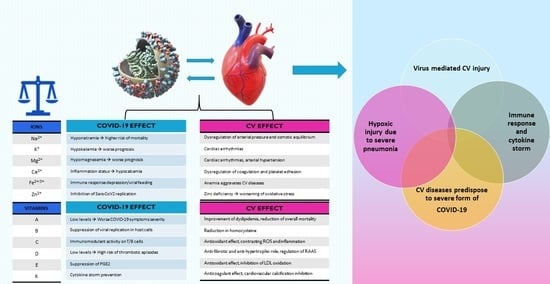
:1. Introduction
2. Coronavirus Disease 2019 and Cardiovascular System
3. Coronavirus Disease 2019 and Cardiovascular Diseases: The Role of Ions
3.1. Sodium
3.2. Magnesium
3.3. Potassium
3.4. Calcium
3.5. Iron
3.6. Zinc
4. Coronavirus Disease 2019 and Cardiovascular Diseases: The Role of Vitamins
4.1. Vitamin A
4.2. Vitamin B
4.3. Vitamin C
4.4. Vitamin D
4.5. Vitamin E
4.6. Vitamin K
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vest, A.R.; Chan, M.; Deswal, A.; Givertz, M.M.; Lekavich, C.; Lennie, T.; Litwin, S.E.; Parsly, L.; Rodgers, J.E.; Rich, M.W.; et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J. Card. Fail. 2019, 25, 380–400. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular and Stroke Nursing; Council on Hypertension; et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Pourfridoni, M.; Abbasnia, S.M.; Shafaei, F.; Razaviyan, J.; Heidari-Soureshjani, R. Fluid and Electrolyte Disturbances in COVID-19 and Their Complications. Biomed. Res. Int. 2021, 2021, 6667047. [Google Scholar] [CrossRef]
- Maestrini, V.; Birtolo, L.I.; Francone, M.; Galardo, G.; Galea, N.; Severino, P.; Alessandri, F.; Colaiacomo, M.C.; Cundari, G.; Chimenti, C.; et al. Cardiac involvement in consecutive unselected hospitalized COVID-19 population: In-hospital evaluation and one-year follow-up. Int. J. Cardiol. 2021, 339, 235–242. [Google Scholar] [CrossRef]
- Azevedo, R.B.; Botelho, B.G.; Hollanda, J.V.G.; Ferreira, L.V.L.; Junqueira de Andrade, L.Z.; Oei, S.S.M.L.; Mello, T.S.; Muxfeldt, E.S. COVID-19 and the cardiovascular system: A comprehensive review. J. Hum. Hypertens. 2021, 35, 4–11. [Google Scholar] [CrossRef]
- Mitacchione, G.; Schiavone, M.; Curnis, A.; Arca, M.; Antinori, S.; Gasperetti, A.; Mascioli, G.; Severino, P.; Sabato, F.; Caracciolo, M.M.; et al. Impact of prior statin use on clinical outcomes in COVID-19 patients: Data from tertiary referral hospitals during COVID-19 pandemic in Italy. J. Clin. Lipidol. 2021, 15, 68–78. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, X.; Jian, J.; Chen, X.; Yin, K. Cardiovascular disease in patients with COVID-19: Evidence from cardiovascular pathology to treatment. Acta Biochim. Biophys. Sin. 2021, 53, 273–282. [Google Scholar] [CrossRef]
- Chilazi, M.; Duffy, E.Y.; Thakkar, A.; Michos, E.D. COVID and Cardiovascular Disease: What We Know in 2021. Curr. Atheroscler. Rep. 2021, 23, 37. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Billig Rose, E.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network-United States, March–June 2020. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef]
- Adams, J.G.; Walls, R.M. Supporting the health care workforce during the COVID-19 global epidemic. JAMA 2020, 323, 1439–1440. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef] [Green Version]
- Cenko, E.; Badimon, L.; Bugiardini, R.; Claeys, M.J.; De Luca, G.; de Wit, C.; Derumeaux, G.; Dorobantu, M.; Duncker, D.J.; Eringa, E.C.; et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc. Res. 2021, 117, 2705–2729. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Deng, S.Q.; Peng, H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Coats, A.J.S.; Zheng, Z.; Adamo, M.; Ambrosio, G.; Anker, S.D.; Butler, J.; Xu, D.; Mao, J.; Khan, M.S.; et al. Management of heart failure patients with COVID-19: A joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 941–956. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, Y.; Smith, S.W.; Sexter, A.; Thordsen, S.E.; Bruen, C.A.; Carlson, M.D.; Dodd, K.W.; Driver, B.E.; Hu, Y.; Jacoby, K.; et al. Type 1 and 2 myocardial infarction and myocardial injury: Clinical transition to high-sensitivity cardiac troponin I. Am. J. Med. 2017, 130, 1431–1439.e4. [Google Scholar] [CrossRef] [Green Version]
- Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Jangaard, N.; Hosbond, S.; Diederichsen, A.C.; Thygesen, K.; Mickley, H. Clinical characteristics and outcomes of patients with myocardial infarction, myocardial injury, and non elevated troponins. Am. J. Med. 2016, 129, 446.e5–446.e21. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Chimenti, C.; Magnocavallo, M.; Ballatore, F.; Bernardini, F.; Alfarano, M.; Della Rocca, D.G.; Severino, P.; Lavalle, C.; Francesco, F.; Frustaci, A. Prevalence and Clinical Implications of COVID-19 Myocarditis. Card. Electrophysiol. Clin. 2022, 14, 53–62. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Taghizadeh, H.; Akbari, A.; Inabadi, M.; Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti Infect. Ther. 2021, 19, 345–357. [Google Scholar] [CrossRef]
- Kochi, A.N.; Tagliari, A.P.; Forleo, G.B.; Fassini, G.M.; Tondo, C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Magnocavallo, M.; Lavalle, C.; Romero, J.; Forleo, G.B.; Tarantino, N.; Chimenti, C.; Alviz, I.; Gamero, M.T.; Garcia, M.J.; et al. Evidence of systemic endothelial injury and microthrombosis in hospitalized COVID-19 patients at different stages of the disease. J. Thromb. Thrombolysis 2021, 51, 571–576. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Walter, E.J.; Hanna-Jumma, S.; Carraretto, M.; Forni, L. The pathophysiological basis and consequences of fever. Crit. Care 2016, 20, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, H.B. Hyperthermia. N. Engl. J. Med. 1993, 329, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xie, L.; Liu, R.; Yang, J.; Liu, F.; Wu, K.; Chen, L.; Hou, W.; Feng, Y.; Zhu, C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J. Med. Virol. 2020, 92, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Magnocavallo, M.; Maraone, A.; Notari, C.; Papisca, I.; Mancone, M.; Fedele, F. Clinical Support through Telemedicine in Heart Failure Outpatients during the COVID-19 Pandemic Period: Results of a 12-Months Follow Up. J. Clin. Med. 2022, 11, 2790. [Google Scholar] [CrossRef]
- Piro, A.; Magnocavallo, M.; Della Rocca, D.G.; Neccia, M.; Manzi, G.; Mariani, M.V.; Straito, M.; Bernardini, A.; Severino, P.; Iannucci, G.; et al. Management of cardiac implantable electronic device follow-up in COVID-19 pandemic: Lessons learned during Italian lockdown. J. Cardiovasc. Electrophysiol. 2020, 31, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Magnocavallo, M.; Vetta, G.; Bernardini, A.; Piro, A.; Mei, M.C.; Di Iorio, M.; Mariani, M.V.; Della Rocca, D.G.; Severino, P.; Quaglione, R.; et al. Impact of COVID-19 Pandemic on Cardiac Electronic Device Management and Role of Remote Monitoring. Card. Electrophysiol. Clin. 2022, 14, 125–131. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Saglietto, A.; D’Ascenzo, F.; Marini, C.; Schiavone, M.; Ghionzoli, N.; Pirrotta, F.; Troiano, F.; Cannillo, M.; et al. Reduction in heart failure hospitalization rate during coronavirus disease 19 pandemic outbreak. ESC Heart Fail. 2020, 7, 4182–4188. [Google Scholar] [CrossRef]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol. Dial. Transplant. 2014, 29 (Suppl. 2), i1–i39. [Google Scholar] [CrossRef] [Green Version]
- Hoorn, E.J.; Betjes, M.G.; Weigel, J.; Zietse, R. Hypernatraemia in critically ill patients: Too little water and too much salt. Nephrol. Dial. Transplant. 2008, 23, 1562–1568. [Google Scholar] [CrossRef] [Green Version]
- Frontera, J.A.; Valdes, E.; Huang, J.; Lewis, A.; Lord, A.S.; Zhou, T.; Kahn, D.E.; Melmed, K.; Czeisler, B.M.; Yaghi, S.; et al. Prevalence and Impact of Hyponatremia in Patients With Coronavirus Disease 2019 in New York City. Crit. Care Med. 2020, 48, e1211–e1217. [Google Scholar] [CrossRef]
- Cuesta, M.; Thompson, C.J. The syndrome of inappropriate antidiuresis (SIAD). Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 175–187. [Google Scholar] [CrossRef]
- Cuesta, M.; Slattery, D.; Goulden, E.L.; Gupta, S.; Tatro, E.; Sherlock, M.; Tormey, W.; O’Neill, S.; Thompson, C.J. Hyponatraemia in patients with community-acquired pneumonia; prevalence and aetiology, and natural history of SIAD. Clin. Endocrinol. 2019, 90, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, A.; Rysz, S.; Sjöström, H.; Höybye, C. Electrolyte and acid-base imbalance in severe COVID-19. Endocr. Connect. 2021, 10, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Swart, R.M.; Hoorn, E.J.; Betjes, M.G.; Zietse, R. Hyponatremia and Inflammation: The Emerging Role of Interleukin-6 in Osmoregulation. Nephron. Physiol. 2011, 118, p45–p51. [Google Scholar] [CrossRef] [PubMed]
- Berni, A.; Malandrino, D.; Parenti, G.; Maggi, M.; Poggesi, L.; Peri, A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: May all fit together? J. Endocrinol. Invest. 2020, 43, 1137–1139. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Uppal, N.N.; Sharma, P.; Khanin, Y.; Shah, H.H.; Malieckal, D.A.; Bellucci, A.; Sachdeva, M.; Rondon-Berrios, H.; Jhaveri, K.D.; et al. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol. Dial. Transplant. 2021, 36, 1135–1138. [Google Scholar] [CrossRef]
- Olsen, M.H.; Møller, M.; Romano, S.; Andersson, J.; Mlodzinski, E.; Raines, N.H.; Sherak, R.; Jeppesen, A.N. Association Between ICU-Acquired Hypernatremia and In-Hospital Mortality: Data From the Medical Information Mart for Intensive Care III and the Electronic ICU Collaborative Research Database. Crit. Care Explor. 2020, 2, e0304. [Google Scholar] [CrossRef]
- Tzoulis, P.; Waung, J.A.; Bagkeris, E.; Hussein, Z.; Biddanda, A.; Cousins, J.; Dewsnip, A.; Falayi, K.; McCaughran, W.; Mullins, C.; et al. Dysnatremia is a Predictor for Morbidity and Mortality in Hospitalized Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, 1637–1648. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.Y. Prevalence of hyponatremia among older inpatients in a general hospital. Eur. Geriatr. Med. 2020, 11, 685–692. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in Infectious Diseases in Older People. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Rochelson, B.; Dowling, O.; Schwartz, N.; Metz, C.N. Magnesium sulfate suppresses inflammatory responses by human umbilical vein endothelial cells (HuVECs) through the NFkappaB pathway. J. Reprod. Immunol. 2007, 73, 101–107. [Google Scholar] [CrossRef]
- Kao, M.C.; Jan, W.C.; Tsai, P.S.; Wang, T.Y.; Huang, C.J. Magnesium sulfate mitigates lung injury induced by bilateral lower limb ischemia-reperfusion in rats. J. Surg. Res. 2011, 171, e97–e106. [Google Scholar] [CrossRef] [PubMed]
- Shimosawa, T.; Takano, K.; Ando, K.; Fujita, T. Magnesium inhibits norepinephrine release by blocking N-type calcium channels at peripheral sympathetic nerve endings. Hypertension 2004, 44, 897–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachnas, M.A.; Akbar, M.I.A.; Dachlan, E.G.; Dekker, G. The role of magnesium sulfate (MgSO4) in fetal neuroprotection. J. Matern. Fetal. Neonatal. Med. 2021, 34, 966–978. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Magnesium and Vitamin D Deficiency as a Potential Cause of Immune Dysfunction, Cytokine Storm and Disseminated Intravascular Coagulation in COVID-19 patients. Mo. Med. 2021, 118, 68–73. [Google Scholar] [PubMed]
- Severino, P.; Netti, L.; Mariani, M.V.; Maraone, A.; D’Amato, A.; Scarpati, R.; Infusino, F.; Pucci, M.; Lavalle, C.; Maestrini, V.; et al. Prevention of Cardiovascular Disease: Screening for Magnesium Deficiency. Cardiol. Res. Pract. 2019, 2019, 4874921. [Google Scholar] [CrossRef] [Green Version]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Kim, J.E.; Shin, C.S.; Lee, Y.C.; Lee, H.S.; Ban, M.; Kim, S.Y. Beneficial effect of intravenous magnesium during endoscopic submucosal dissection for gastric neoplasm. Surg. Endosc. 2015, 29, 3795–3802. [Google Scholar] [CrossRef]
- Altura, B.M.; Altura, B.T.; Carella, A.; Gebrewold, A.; Murakawa, T.; Nishio, A. Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can. J. Physiol. Pharmacol. 1987, 65, 729–745. [Google Scholar] [CrossRef]
- Ko, E.A.; Han, J.; Jung, I.D.; Park, W.S. Physiological roles of K+ channels in vascular smooth muscle cells. J. Smooth Muscle Res. 2008, 44, 65–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Cheng, T.P.; Altura, B.T.; Altura, B.M. Mg2+ and caffeine-induced intracellular Ca2+ release in human vascular endothelial cells. Br. J. Pharmacol. 1993, 109, 291–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quilliot, D.; Bonsack, O.; Jaussaud, R.; Mazur, A. Dysmagnesemia in COVID-19 cohort patients: Prevalence and associated factors. Magnes. Res. 2020, 33, 114–122. [Google Scholar] [CrossRef]
- Alamdari, N.M.; Afaghi, S.; Rahimi, F.S.; Tarki, F.E.; Tavana, S.; Zali, A.; Fathi, M.; Besharat, S.; Bagheri, L.; Pourmotahari, F.; et al. Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran. Tohoku J. Exp. Med. 2020, 252, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Wiles, M.E.; Wagner, T.L.; Weglicki, W.B. Effect of acute magnesium deficiency (MgD) on aortic endothelial cell (EC) oxidant production. Life Sci. 1997, 60, 221–236. [Google Scholar] [CrossRef]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Ferré, S.; Maier, J.A. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved? Magnes. Res. 2008, 21, 58–64. [Google Scholar]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; Infusino, F.; Maestrini, V.; Mancone, M.; Fedele, F. Myocardial Ischemia and Diabetes Mellitus: Role of Oxidative Stress in the Connection between Cardiac Metabolism and Coronary Blood Flow. J. Diabetes Res. 2019, 2019, 9489826. [Google Scholar] [CrossRef]
- Cuijpers, I.; Simmonds, S.J.; van Bilsen, M.; Czarnowska, E.; González Miqueo, A.; Heymans, S.; Kuhn, A.R.; Mulder, P.; Ratajska, A.; Jones, E.A.V.; et al. Microvascular and lymphatic dysfunction in HFpEF and its associated comorbidities. Basic Res. Cardiol. 2020, 115, 39. [Google Scholar] [CrossRef]
- Rush, C.J.; Berry, C.; Oldroyd, K.G. Prevalence of Coronary Artery Disease and Coronary Microvascular Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2021, 6, 1130–1143. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; D’Amato, A.; Prosperi, S.; Fanisio, F.; Birtolo, L.I.; Costi, B.; Netti, L.; Chimenti, C.; Lavalle, C.; Maestrini, V.; et al. Myocardial Tissue Characterization in Heart Failure with Preserved Ejection Fraction: From Histopathology and Cardiac Magnetic Resonance Findings to Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 7650. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Phoenix, F.A.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Reduced Plasma Magnesium Levels in Type-1 Diabetes Associate with Prothrombotic Changes in Fibrin Clotting and Fibrinolysis. Thromb. Haemost. 2020, 120, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çiçek, G.; Açıkgoz, S.K.; Yayla, Ç; Kundi, H.; İleri, M. Magnesium as a predictor of acute stent thrombosis in patients with ST-segment elevation myocardial infarction who underwent primary angioplasty. Coron. Artery Dis. 2016, 27, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Mussoni, L.; Sironi, L.; Tedeschi, L.; Calvio, A.M.; Colli, S.; Tremoli, E. Magnesium inhibits arterial thrombi after vascular injury in rat: In vivo impairment of coagulation. Thromb. Haemost. 2001, 86, 1292–1295. [Google Scholar] [PubMed]
- Crisponi, G.; Nurchi, V.M.; Cappai, R.; Zoroddu, M.A.; Gerosa, C.; Piras, M.; Faa, G.; Fanni, D. The Potential Clinical Properties of Magnesium. Curr. Med. Chem. 2021, 28, 7295–7311. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Sato, T.; Hashimoto, Y.; Yoshioka, H.; Ohtomo, N.; Ishihara, H.; Matsuki, A. Relaxant effect of magnesium and zinc on histamine-induced bronchoconstriction in dogs. Crit. Care Med. 1999, 27, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Landon, R.A.; Young, E.A. Role of magnesium in regulation of lung function. J. Am. Diet. Assoc. 1993, 93, 674–677. [Google Scholar] [CrossRef]
- Güzel, A.; Dogan, E.; Türkçü, G.; Kuyumcu, M.; Kaplan, I.; Çelik, F.; Yıldırım, Z.B. Dexmedetomidine and magnesium sulfate: A good combination treatment for acute lung injury? J. Invest. Surg. 2019, 32, 331–342. [Google Scholar] [CrossRef]
- Rastegar, A.; Soleimani, M. Hypokalaemia and hyperkalaemia. Postgrad. Med. J. 2001, 77, 759–764. [Google Scholar] [CrossRef]
- McDonough, A.A.; Youn, J.H. Potassium Homeostasis: The Knowns, the Unknowns, and the Health Benefits. Physiology 2017, 32, 100–111. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clase, C.M.; Carrero, J.-J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020, 97, 42–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, A.; Rabito, G.; Pironi, M.; Bissig, M.; Parlato, S.; Andreocchi, L.; Bianchi, G.; Poretti Guigli, M.; Llamas, M.; Monotti, R.; et al. Hypokalaemia in hospitalised patients. Swiss Med. Wkly 2016, 146, w14320. [Google Scholar] [CrossRef]
- Paice, B.J.; Paterson, K.R.; Onyanga-Omara, F.; Donnelly, T.; Gray, J.M.; Lawson, D.H. Record linkage study of hypokalaemia in hospitalized patients. Postgrad. Med. J. 1986, 62, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Sarvazad, H.; Cahngaripour, S.H.; Roozbahani, N.E.; Izadi, B. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. New Microbes New Infect. 2020, 38, 100807. [Google Scholar] [CrossRef]
- Rosenthal, N.; Cao, Z.; Gundrum, J.; Sianis, J.; Safo, S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Network Open 2020, 3, e2029058. [Google Scholar] [CrossRef]
- Mallow, P.J.; Belk, K.W.; Topmiller, M.; Hooker, E.A. Outcomes of hospitalized COVID-19 patients by risk factors: Results from a United States hospital claims database. J. Health Econ. Outcomes Res. 2020, 7, 165–175. [Google Scholar] [CrossRef]
- Chen, D.; Li, X.; Song, Q.; Hu, C.; Su, F.; Dai, J.; Ye, Y.; Huang, J.; Zhang, X. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Network Open 2020, 3, e2011122. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Weng, H.; Yang, F.; Jin, H.; Fan, F.; Zheng, X.; Yang, H.; Li, H.; Zhang, Y.; et al. Association between average plasma potassium levels and 30-day mortality during hospitalization in patients with COVID-19 in Wuhan, China. Int. J. Med. Sci. 2021, 18, 736–743. [Google Scholar] [CrossRef]
- Tezcan, M.E.; Dogan Gokce, G.; Sen, N.; Zorlutuna Kaymak, N.; Ozer, R.S. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized coronavirus disease 2019 patients. New Microbes New Infect. 2020, 37, 100753. [Google Scholar] [CrossRef] [PubMed]
- Szoke, D.; Caruso, S.; Aloisio, E.; Pasqualetti, S.; Dolci, A.; Panteghini, M. Serum potassium concentrations in COVID-19. Clin. Chim. Acta 2021, 512, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Muhanna, D.; Arnipalli, S.R.; Kumar, S.B.; Ziouzenkova, O. Osmotic Adaptation by Na(+)-Dependent Transporters and ACE2: Correlation with Hemostatic crisis in COVID-19. Biomedicines 2020, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Silhol, F.; Sarlon, G.; Deharo, J.-C.; Vaïsse, B. Downregulation of ACE2 induces overstimulation of the renin–angiotensin system in COVID-19: Should we block the renin–angiotensin system? Hypertens Res. 2020, 43, 854–856. [Google Scholar] [CrossRef]
- de Melom, I.S.; Sabino-Silva, R.; Cunha, T.M.; Goulart, L.R.; Reis, W.L.; Jardim, A.C.G.; Shetty, A.K.; de Castro, O.W. Hydroelectrolytic disorder in COVID-19 patients: Evidence supporting the Involvement of subfornical organ and paraventricular nucleus of the Hypothalamus. Neurosci. Biobehav. Rev. 2021, 124, 216–223. [Google Scholar] [CrossRef]
- Alfano, G.; Ferrari, A.; Fontana, F.; Perrone, R.; Mori, G.; Ascione, E.; Magistroni, R.; Venturi, G.; Pederzoli, S.; Margiotta, G.; et al. Hypokalemia in patients with COVID-19. Clin. Exp. Nephrol. 2021, 25, 1–409. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, Y.; Huang, Q.; Fu, S.; Li, X.; Lin, B.; Xu, A.; Chen, J. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. 2021, 22, 52. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Bowling, C.B.; Pitt, B.; Ahmed, M.I.; Aban, I.B.; Sanders, P.W.; Mujib, M.; Campbell, R.C.; Love, T.E.; Aronow, W.S.; Allman, R.M.; et al. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: Findings from propensity-matched studies. Circ. Heart Fail. 2010, 3, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Goyal, A.; Spertus, J.A.; Gosch, K.; Venkitachalam, L.; Jones, P.G.; Van den Berghe, G.; Kosiborod, M. Serum potassium levels and mortality in acute myocardial infarction. JAMA 2012, 307, 157–164. [Google Scholar] [CrossRef]
- Coromilas, E.J.; Kochav, S.; Goldenthal, I.; Biviano, A.; Garan, H.; Goldbarg, S.; Kim, J.H.; Yeo, I.; Tracy, C.; Ayanian, S.; et al. Worldwide survey of COVID-19 associated arrhythmias. Circ. Arrhyth. Electrophysiol. 2021, 14, e009458. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.; Poterucha, T.J.; Jain, S.S.; Sayer, G.; Raikhelkar, J.; Fried, J.; Clerkin, K.; Griffin, J.; DeFilippis, E.M.; Gupta, A.; et al. The prognostic value of electrocardiogram at presentation to emergency department in patients with COVID-19. Mayo Clin. Proc. 2020, 95, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Carson-Chahhoud, K.; Kolahi, A.A.; Safiri, S. Epidemiology, prognosis and management of potassium disorders in Covid-19. Rev. Med. Virol. 2022, 32, e2262. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; Formenti, A.M.; Rovere-Querini, P.; Carlucci, M.; Conte, C.; Ciceri, F.; Zangrillo, A.; Giustina, A. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine 2020, 68, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, F.; Brivio, R.; Casati, M.; Cavallero, A.; Contro, E.; Brambilla, P. Low levels of total and ionized calcium in blood of COVID-19 patients. Clin. Chem. Lab. Med. 2020, 58, e171–e173. [Google Scholar] [CrossRef]
- di Filippo, L.; Formenti, A.M.; Doga, M.; Frara, S.; Rovere-Querini, P.; Bosi, E.; Carlucci, M.; Giustina, A. Hypocalcemia is a distinctive biochemical feature of hospitalized COVID-19 patients. Endocrine 2021, 71, 9–13. [Google Scholar] [CrossRef]
- Pal, R.; Ram, S.; Zohmangaihi, D.; Biswas, I.; Suri, V.; Yaddanapudi, L.N.; Malhotra, P.; Soni, S.L.; Puri, G.D.; Bhalla, A.; et al. High Prevalence of Hypocalcemia in Non-severe COVID-19 Patients: A Retrospective Case-Control Study. Front. Med. 2021, 7, 590805. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Nan, D.; Fernandez-Ayala, M.; García-Unzueta, M.; Hernández-Hernández, M.A.; López-Hoyos, M.; Muñoz-Cacho, P.; Olmos, J.M.; Gutiérrez-Cuadra, M.; Ruiz-Cubillán, J.J.; et al. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J Clin. Endocrinol. Metab. 2021, 106, e1343–e1353. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef]
- Zhou, Y.; Frey, T.K.; Yang, J.J. Viral calciomics: Interplays between Ca2+ and virus. Cell Calcium. 2009, 46, 1–17. [Google Scholar] [CrossRef]
- Singh, S.; Dodt, J.; Volkers, P.; Hethershaw, E.; Philippou, H.; Ivaskevicius, V.; Imhof, D.; Oldenburg, J.; Biswas, A. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Sci. Rep. 2019, 9, 11324. [Google Scholar] [CrossRef] [PubMed]
- Terrar, D.A. Calcium signaling in the heart. Adv. Exp. Med. Biol. 2020, 1131, 395–443. [Google Scholar] [CrossRef] [PubMed]
- Hoydal, M.A.; Kirkeby-Garstad, I.; Karevold, A.; Wiseth, R.; Haaverstad, R.; Wahba, A.; Stølen, T.L.; Contu, R.; Condorelli, G.; Ellingsen, Ø.; et al. Human cardiomyocyte calcium handling and transverse tubules in mid-stage of post-myocardial-infarction heart failure. ESC Heart Fail. 2018, 5, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Du, B.; Li, J.; Wang, S.; Wang, X.; Guo, M.; Yang, B.; Si, D.; Bai, O. D-dimer surge and coagulation disorders in COVID-19 related pneumonia patients with cardiac injury: A case series. Medicine 2020, 99, e21513. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Ogawa, H. Perspective on fibrinolytic therapy in COVID-19: The potential of inhalation therapy against suppressed-fibrinolytic-type DIC. J. Intensive Care 2020, 8, 71. [Google Scholar] [CrossRef]
- Liu, J.; Han, P.; Wu, J.; Gong, J.; Tian, D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J. Infect. Public Health 2020, 13, 1224–1228. [Google Scholar] [CrossRef]
- Sun, J.K.; Zhang, W.H.; Zou, L.; Liu, Y.; Li, J.J.; Kan, X.H.; Dai, L.; Shi, Q.K.; Yuan, S.T.; Yu, W.K.; et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging 2020, 12, 11287–11295. [Google Scholar] [CrossRef]
- Qi, X.; Kong, H.; Ding, W.; Wu, C.; Ji, N.; Huang, M.; Li, T.; Wang, X.; Wen, J.; Wu, W.; et al. Abnormal Coagulation Function of Patients With COVID-19 Is Significantly Related to Hypocalcemia and Severe Inflammation. Front. Med. 2021, 8, 638194. [Google Scholar] [CrossRef]
- Bennouar, S.; Cherif, A.B.; Kessira, A.; Bennouar, D.E.; Abdi, S. Vitamin D Deficiency and Low Serum Calcium as Predictors of Poor Prognosis in Patients with Severe COVID-19. J. Am. Coll. Nutr. 2021, 40, 104–110. [Google Scholar] [CrossRef]
- Linli, Z.; Chen, Y.; Tian, G.; Guo, S.; Fei, Y. Identifying and quantifying robust risk factors for mortality in critically ill patients with COVID-19 using quantile regression. Am. J. Emerg. Med. 2020, 45, 345–351. [Google Scholar] [CrossRef] [PubMed]
- El-Kurdi, B.; Khatua, B.; Rood, C.; Snozek, C.; Cartin-Ceba, R.; Singh, V.P.; Lipotoxicity in COVID-19 Study Group. Mortality From Coronavirus Disease 2019 Increases With Unsaturated Fat and May Be Reduced by Early Calcium and Albumin Supplementation. Gastroenterology 2020, 159, 1015–1018.e4. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Asp. Med. 2020, 75, 100864. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Maier, J.; Gorki, A.D.; Huber, K.V.; Sharif, O.; Starkl, P.; Saluzzo, S.; Quattrone, F.; Gawish, R.; Lakovits, K.; et al. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat. Immunol. 2016, 17, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.C.; Acton, R.T. Hepcidin, iron, and bacterial infection. Vitam. Horm. 2019, 110, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Takasawa, K. Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease. Nutrients 2018, 10, 1173. [Google Scholar] [CrossRef] [Green Version]
- Taneri, P.E.; Gómez-Ochoa, S.A.; Llanaj, E.; Raguindin, P.F.; Rojas, L.Z.; Roa-Díaz, Z.M.; Salvador, D., Jr.; Groothof, D.; Minder, B.; Kopp-Heim, D.; et al. Anemia and iron metabolism in COVID-19: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 763–773. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yu, X.; Hu, D.; Bao, M.; Liu, H.; Zhou, J.; Jiang, H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020, 80, 639–645. [Google Scholar] [CrossRef]
- Fan, B.E.; Chong, V.C.L.; Chan, S.S.W.; Lim, G.H.; Lim, K.G.E.; Tan, G.B.; Mucheli, S.S.; Kuperan, P.; Ong, K.H. Hematologic parameters in patients with COVID-19 infection. Am. J. Hematol. 2020, 95, E131–E134. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.; Das, J.K.; Ismail, T.; Wahid, M.; Saeed, W.; Bhutta, Z.A. Nutritional perspectives for the prevention and mitigation of COVID-19. Nutr. Rev. 2021, 79, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, S.; Nekhai, S.; Liu, S. Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic Against Viral Survival. Curr. Clin. Microbiol. Rep. 2020, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- de Pontual, L. Fer et prédisposition aux infections [Iron and susceptibility to infections]. Arch. Pediatr. 2017, 24, 5S14–5S17. [Google Scholar] [CrossRef]
- Jayaweera, J.A.A.S.; Reyes, M.; Joseph, A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci. Rep. 2019, 9, 12637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinkove, R.; McQuilten, Z.; Adler, J.; Agar, M.R.; Blyth, E.; Cheng, A.C.; Conyers, R.; Haeusler, G.M.; Hardie, C.; Jackson, C.; et al. Managing haematology and oncology patients during the COVID-19 pandemic: Interim consensus guidance. Med. J. Aust. 2020, 212, 481–489. [Google Scholar] [CrossRef]
- Iorio, A.; Senni, M.; Barbati, G.; Greene, S.J.; Poli, S.; Zambon, E.; Di Nora, C.; Cioffi, G.; Tarantini, L.; Gavazzi, A.; et al. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: A community-based study. Eur. J. Heart Fail. 2018, 20, 12571266. [Google Scholar] [CrossRef] [Green Version]
- Anand, I.S.; Gupta, P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation 2018, 138, 8098. [Google Scholar] [CrossRef]
- McDonagh, T.; Damy, T.; Doehner, W.; Lam, C.S.P.; Sindone, A.; van der Meer, P.; Cohen-Solal, A.; Kindermann, I.; Manito, N.; Pfister, O.; et al. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: Putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur. J. Heart Fail. 2018, 20, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Rocha, B.M.L.; Cunha, G.J.L.; Falcao Menezes, L.F. The burden of iron deficiency in heart failure: Therapeutic approach. J. Am. Coll. Cardiol. 2018, 71, 782793. [Google Scholar] [CrossRef]
- von Haehling, S.; Jankowska, E.A.; van Veldhuisen, J.; Ponikowski, P.; Anker, S.D. Iron deficiency and cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 659–669. [Google Scholar] [CrossRef]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am. Heart J. 2013, 165, 575582.e3. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010, 31, 18721880. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, D.H.; Kragten, J.A.; Means, M.E.; van Ofwegen-Hanekamp, C.E.E.; Klaarwater, C.C.R.; Spanjers, M.H.A.; Hendrick, R.; van Deursen, C.T.B.M.; Brunner-La Rocca, H.P. Acute heart failure and iron deficiency: A prospective, multicentre, observational study. ESC Heart Fail. 2022, 9, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Sierpinski, R.; Josiak, K.; Suchocki, T.; Wojtas-Polc, K.; Mazur, G.; Butrym, A.; Rozentryt, P.; van der Meer, P.; Comin-Colet, J.; von Haehling, S.; et al. High soluble transferrin receptor in patients with heart failure: A measure of iron deficiency and a strong predictor of mortality. Eur. J. Heart Fail. 2020, 23, 919–932. [Google Scholar] [CrossRef]
- Fitzsimons, S.; Poppe, K.K.; Choi, Y.; Devlin, G.; Lund, M.; Lam, C.S.; Troughton, R.; Richards, A.M.; Doughty, R.N. Relationship between soluble transferrin receptor and clinical outcomes in patients with Heart Failure According to Ejection Fraction Phenotype: The New Zealand PEOPLE Study. J. Card. Fail. 2022, 28, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Kirwan, B.A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Göhring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 18951904. [Google Scholar] [CrossRef]
- López-Vilella, R.; Lozano-Edo, S.; Arenas Martín, P.; Jover-Pastor, P.; Ezzitouny, M.; Sorolla Romero, J.; Calvo Asensio, M.; Martínez-Solé, J.; Guerrero Cervera, B.; Sánchez Martínez, J.C.; et al. Impact of intravenous ferric carboxymaltose on heart failure with preserved and reduced ejection fraction. ESC Heart Fail. 2022, 9, 133–145. [Google Scholar] [CrossRef]
- Tu, S.J.; Elliott, A.D.; Hanna-Rivero, N.; Gallagher, C.; Mishima, R.S.; Lyrtzis, E.; Wlochowicz, D.; Clarke, N.A.; Roberts-Thomson, K.C.; Stokes, M.B.; et al. Rationale and design of the IRON-AF study: A double-blind, randomised, placebo-controlled study to assess the effect of intravenous ferric carboxymaltose in patients with atrial fibrillation and iron deficiency. BMJ Open 2021, 11, e047642. [Google Scholar] [CrossRef]
- Vinke, P.; Koudstaal, T.; Muskens, F.; van den Bosch, A.; Balvers, M.; Poland, M.; Witkamp, R.F.; van Norren, K.; Boomars, K.A. Prevalence of Micronutrient Deficiencies and Relationship with Clinical and Patient-Related Outcomes in Pulmonary Hypertension Types I and IV. Nutrients 2021, 13, 3923. [Google Scholar] [CrossRef]
- Gürgöze, M.T.; Kardys, I.; Akkerhuis, K.M.; Oemrawsingh, R.M.; Groot, H.E.; van der Harst, P.; Umans, V.A.; Kietselaer, B.; Ronner, E.; Lenderink, T.; et al. Relation of Iron Status to Prognosis After Acute Coronary Syndrome. Am. J. Cardiol. 2022, 168, 22–30. [Google Scholar] [CrossRef]
- Meng, H.; Wang, Y.; Ruan, J.; Chen, Y.; Wang, X.; Zhou, F.; Meng, F. Decreased Iron Ion Concentrations in the Peripheral Blood Correlate with Coronary Atherosclerosis. Nutrients 2022, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Prasad, A.S.; Beck, F.W.J.; Sarkar, F.H. Zinc up-regulates NF-κB activation via phosphorylation of IκB in HUT-78 (Th0) cells. FEBS Lett. 2007, 581, 4507–4511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases review-article. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, P.M. Endothelial dysfunction-The first step toward coronary arteriosclerosis. Circ. J. 2009, 73, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; D’Amato, A.; Prosperi, S.; Magnocavallo, M.; Mariani, M.V.; Netti, L.; Birtolo, L.I.; De Orchi, P.; Chimenti, C.; Maestrini, V.; et al. Potential Role of eNOS Genetic Variants in Ischemic Heart Disease Susceptibility and Clinical Presentation. J. Cardiovasc. Dev. Dis. 2021, 8, 116. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Karim, M.M.; Sultana, S.; Sultana, R.; Rahman, M.T. Possible Benefits of Zinc supplement in CVD and COVID-19 Comorbidity. J. Infect. Public Health 2021, 14, 1686–1692. [Google Scholar] [CrossRef]
- Yi, T.; Vick, J.S.; Vecchio, M.J.H.; Begin, K.J.; Bell, S.P.; Delay, R.J.; Palmer, B.M. Identifying cellular mechanisms of zinc-induced relaxation in isolated cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H706–H715. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/Mas axis of the renin-angiotensin system: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [Green Version]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Rahman, M.T.; Idid, S.Z. Can Zn Be a Critical Element in COVID-19 Treatment? Biol. Trace Elem. Res. 2020, 199, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Mbise, R.; Spiegelman, D.; Hertzmark, E.; Fataki, M.; Peterson, K.E.; Ndossi, G.; Fawzi, W.W. Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth. Pediatrics 2002, 109, E6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saleh, I.; Alrushud, N.; Alnuwaysir, H.; Elkhatib, R.; Shoukri, M.; Aldayel, F.; Bakheet, R.; Almozaini, M. Essential metals, vitamins and antioxidant enzyme activities in COVID-19 patients and their potential associations with the disease severity. Biometals 2022, 35, 125–145. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Lietz, G. Vitamin A in resistance to and recovery from infection: Relevance to SARS-CoV2. Br. J. Nutr. 2021, 126, 1663–1672. [Google Scholar] [CrossRef]
- Wang, N.; Ru, Y.; Yang, Z.; Sun, C.; Li, S.; Min, Y.; Zhao, X.; Lu, Y.; Hsing, A.W.; Zhu, S. Metabolomic Profiles of Plasma Retinol-Associated Dyslipidemia in Men and Women. Front. Nutr. 2021, 8, 740435. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Association between serum retinol and overall and cause-specific mortality in a 30-year prospective cohort study. Nat. Commun. 2021, 12, 6418. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, L.M.J. Can vitamin B12 be an adjuvant to COVID-19 treatment? GSC Biol. Pharm. Sci. 2020, 11, 1–5. [Google Scholar] [CrossRef]
- Robinson, K.; Mayer, E.L.; Miller, D.P.; Green, R.; van Lente, F.; Gupta, A.; Kottke-Marchant, K.; Savon, S.R.; Selhub, J.; Nissen, S.E.; et al. Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation 1995, 92, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Perry, I.J.; Refsum, H.; Morris, R.W.; Ebrahim, S.B.; Ueland, P.M.; Shaper, A.G. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet 1995, 346, 1395–1398. [Google Scholar] [CrossRef]
- Eshak, E.S.; Arafa, A.E. Thiamine deficiency and cardiovascular science. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 965–972. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Kenney, W.L. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr. Rev. 2017, 75, 61–70. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.Y.; Qin, Y.Y.; Yu, F.F.; Zhou, Y.H. Association between B vitamins supplementation and risk of cardiovascular outcomes: A cumulative meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e107060. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M. A cohort study to evaluate the effect of combination Vitamin D, Magnesium and Vitamin B12 (DMB) on progression to severe outcome in older COVID-19 patients. Nutrition 2020, 79, 111017. [Google Scholar] [CrossRef]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Fu, G.; Yao, Q.; Cheng, G. Relation of thrombomodulin, TFPI and plasma antioxidants in healthy individuals and patients with coronary heart disease. Acta Cardiol. 2008, 63, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Sanguigni, V.; Paola, S.G.; Lo Coco, E.; Lenti, L.; Violi, F. Vitamin C inhibits platelet expression of CD40 ligand. Free Radic. Biol. Med. 2005, 38, 1662–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabet, M.H.; Mohammadi, M.; Ramezani, M.; Khalili, H. Effect of high-dose Ascorbic acid on vasopressor’s requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94–100. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, H.; Lin, K.; Wang, H.; Wei, H.; Ji, B.; Yang, Z.; Peng, C.; Xiao, X.; Deng, H. 1,25(OH)2 D3 improves cardiac dysfunction, hypertrophy, and fibrosis through PARP1/SIRT1/mTOR-related mechanisms in type 1 diabetes. Mol. Nutr. Food Res. 2017, 61, 1600338. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Law, C.S.; Grigsby, C.L.; Olsen, K.; Hong, T.T.; Zhang, Y.; Yeghiazarians, Y.; Gardner, D.G. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 2011, 124, 1838–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Invest. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Wengle, B.; Wide, L.; Ljunghall, S. Reduction of blood pressure during long-term treatment with active vitamin D (alphacalcidol) is dependent on plasma renin activity and calcium status. A double-blind, placebo-controlled study. Am. J. Hypertens 1989, 2, 20–25. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Nachtigall, D.; Hansen, C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Kawamura, M.; Owada, M.; Oshima, T.; Murooka, M.; Fujiwara, T.; Hiramori, K. Effectiveness of 1,25-dihydroxyvitamin D supplementation on blood pressure reduction in a pseudohypoparathyroidism patient with high renin activity. Intern. Med. 1999, 38, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Park, C.W.; Oh, Y.S.; Shin, Y.S.; Kim, C.M.; Kim, Y.S.; Kim, S.Y.; Choi, E.J.; Chang, Y.S.; Bang, B.K. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am. J. Kidney Dis. 1999, 33, 73–81. [Google Scholar] [CrossRef]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Shin, H.; Kim, M.J.; Ahn, H.Y.; Kang, S.M.; Yoon, J.W.; Choi, S.H.; Kim, K.W.; Song, J.H.; Choi, S.I.; et al. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: The Korean Longitudinal Study on Health and Aging. J. Clin. Endocrinol. Metab. 2012, 97, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Carvalho, H.; Richard, M.C.; Chouihed, T.; Goffinet, N.; Le Bastard, Q.; Freund, Y.; Kratz, A.; Dubroux, M.; Masson, D.; Figueres, L.; et al. Electrolyte imbalance in COVID-19 patients admitted to the Emergency Department: A case-control study. Intern. Emerg. Med. 2021, 16, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Martino, F.; Zicari, A.M.; Carnevale, R.; Battaglia, S.; Martino, E.; Cammisotto, V.; Peruzzi, M.; De Castro, G.; Duse, M.; et al. Enhanced NOX-2 derived oxidative stress in offspring of patients with early myocardial infarction. Int. J. Cardiol. 2019, 293, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Boscoboinik, D.; Szewczyk, A.; Azzi, A. Alpha-tocopherol (vitamin E) regulates vascular smooth muscle cell proliferation and protein kinase C activity. Arch. Biochem. Biophys. 1991, 286, 264–269. [Google Scholar] [CrossRef]
- Meydani, M.; Kwan, P.; Band, M.; Knight, A.; Guo, W.; Goutis, J.; Ordovas, J. Long-term vitamin E supplementation reduces atherosclerosis and mortality in Ldlr-/- mice, but not when fed Western style diet. Atherosclerosis 2014, 233, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Qin, F.; Yan, C.; Patel, R.; Liu, W.; Dong, E. Vitamins C and E attenuate apoptosis, beta-adrenergic receptor desensitization, and sarcoplasmic reticular Ca2+ ATPase downregulation after myocardial infarction. Free Radic. Biol. Med. 2006, 40, 1827–1842. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhang, R.Y.; Bai, J. An anti-oxidative therapy for ameliorating cardiac injuries of critically ill COVID-19-infected patients. Int. J. Cardiol. 2020, 312, 137–138. [Google Scholar] [CrossRef]
- Role of Vitamin K-Dependent Factors Protein S and GAS6 and TAM Receptors in SARS-CoV-2 Infec-tion and COVID-19-Associated Immunothrombosis. Cells 2020, 9, 2186. [CrossRef]
- Baicus, C.; Stoichitoiu, L.E.; Pinte, L.; Badea, C. Anti-coagulant Protein S in COVID-19: The Low Activity Level Is Probably Secondary. Am. J. Ther. 2021, 28, e139–e140. [Google Scholar] [CrossRef]
- Jaminon, A.M.G.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla pro-tein is an independent predictor of both intimal and meDial. vascular calcification in chronic kidney disease. Sci. Rep. 2020, 10, 6586. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.who.int/health-topics/micronutrients#tab=tab_1 (accessed on 10 July 2022).

| Ion | Relationship among CV Diseases and Ions | Relationship among COVID-19 and Ions | Reference |
|---|---|---|---|
| Sodium (Na+) |
|
| [38,40] |
| Magnesium (Mg++) |
|
| [63,64] |
| Potassium (K+) |
|
| [84,89] |
| Calcium (Ca++) |
|
| [106,114,115] |
| Iron (Fe++/Fe+++) |
|
| [128,129,130,131,133] |
| Zinc (Zn++) |
|
| [158,159] |
| Vitamin | Relationship among CV Diseases and Vitamins | Relationship among COVID-19 and Vitamins | Reference |
|---|---|---|---|
| Vitamin A |
|
| [166,169] |
| Vitamin B |
|
| [171,172,176,178] |
| Vitamin C |
|
| [181,182,186] |
| Vitamin D |
|
| [191,192,193,194,195,196,199] |
| Vitamin E |
|
| [199,201,203,204] |
| Vitamin K |
|
| [208,209,210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severino, P.; D’Amato, A.; Prosperi, S.; Myftari, V.; Labbro Francia, A.; Önkaya, M.; Notari, C.; Papisca, I.; Canuti, E.S.; Yarden Revivo, M.; et al. The Mutual Relationship among Cardiovascular Diseases and COVID-19: Focus on Micronutrients Imbalance. Nutrients 2022, 14, 3439. https://doi.org/10.3390/nu14163439
Severino P, D’Amato A, Prosperi S, Myftari V, Labbro Francia A, Önkaya M, Notari C, Papisca I, Canuti ES, Yarden Revivo M, et al. The Mutual Relationship among Cardiovascular Diseases and COVID-19: Focus on Micronutrients Imbalance. Nutrients. 2022; 14(16):3439. https://doi.org/10.3390/nu14163439
Chicago/Turabian StyleSeverino, Paolo, Andrea D’Amato, Silvia Prosperi, Vincenzo Myftari, Aurora Labbro Francia, Merve Önkaya, Claudia Notari, Ilaria Papisca, Elena Sofia Canuti, Mia Yarden Revivo, and et al. 2022. "The Mutual Relationship among Cardiovascular Diseases and COVID-19: Focus on Micronutrients Imbalance" Nutrients 14, no. 16: 3439. https://doi.org/10.3390/nu14163439