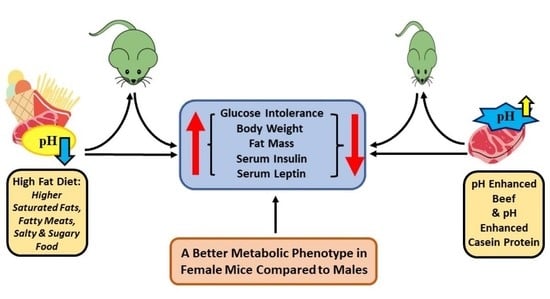
Dietary pH Enhancement Improves Metabolic Outcomes in Diet-Induced Obese Male and Female Mice: Effects of Beef vs. Casein Proteins
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Food Intake of Male and Female Mice during Dietary Intervention
3.2. Sex-Dependent Effects of Diet, Protein Source and pH Enhancement on Weight Gain, Fat Mass and Lean Mass
3.3. Dietary Fat Content and pH Regulate Glucose Clearance, Independent of Sex
3.4. The Effects of Diet and Sex on Serum Metabolic Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected US state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; den Ruijter, H.M.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diabetes Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, J.M.; Divers, J.; Isom, S.; Saydah, S.; Imperatore, G.; Pihoker, C.; Marcovina, S.M.; Mayer-Davis, E.J.; Hamman, R.F.; Dolan, L. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA 2021, 326, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Hinton, W.; McGovern, A.; Coyle, R.; Han, T.S.; Sharma, P.; Correa, A.; Ferreira, F.; de Lusignan, S. Incidence and prevalence of cardiovascular disease in English primary care: A cross-sectional and follow-up study of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC). BMJ Open 2018, 8, e020282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Zinöcker, M.K.; Lindseth, I.A. The Western diet–microbiome-host interaction and its role in metabolic disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V. Ultra-processed diets cause excess calorie intake and weight gain: An inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef] [Green Version]
- de Deus Mendonça, R.; Pimenta, A.M.; Gea, A.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Lopes, A.C.S.; Bes-Rastrollo, M. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2016, 104, 1433–1440. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Isakov, N.F.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef]
- Hariharan, D.; Vellanki, K.; Kramer, H. The Western diet and chronic kidney disease. Curr. Hypertens. Rep. 2015, 17, 16. [Google Scholar] [CrossRef]
- Poti, J.M.; Mendez, M.A.; Ng, S.W.; Popkin, B.M. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am. J. Clin. Nutr. 2015, 101, 1251–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Deus Mendonça, R.; Lopes, A.C.S.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-processed food consumption and the incidence of hypertension in a Mediterranean cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens. 2017, 30, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Carnauba, R.A.; Baptistella, A.B.; Paschoal, V.; Hübscher, G.H. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients 2017, 9, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzorno, J.; Frassetto, L.A.; Katzinger, J. Diet-induced acidosis: Is it real and clinically relevant? Br. J. Nutr. 2010, 103, 1185–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, I.; Maher, T.; Hulter, H.N.; Schambelan, M.; Sebastian, A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983, 24, 670–680. [Google Scholar] [CrossRef] [Green Version]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C., Jr.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Frassetto, L.A.; Morris, R.C., Jr.; Sebastian, A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. Am. J. Physiol.-Ren. Physiol. 2007, 293, F521–F525. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Vilier, A.; Bonnet, F.; Lajous, M.; Balkau, B.; Boutron-Rualt, M.C.; Clavel-Chapelon, F. Dietary acid load and risk of type 2 diabetes: The E3N-EPIC cohort study. Diabetologia 2014, 57, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K. Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br. J. Nutr. 2008, 100, 642–651. [Google Scholar] [CrossRef]
- Winfield, R.D.; Delano, M.J.; Lottenberg, L.; Cendan, J.C.; Moldawer, L.L.; Maier, R.V.; Cuschieri, J. Traditional resuscitative practices fail to resolve metabolic acidosis in morbidly obese patients following severe blunt trauma. J. Trauma 2010, 68, 317. [Google Scholar] [PubMed] [Green Version]
- Williams, R.S.; Kozan, P.; Samocha-Bonet, D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie 2016, 124, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.U.S. Per Capita Availability of Red Meat, Poultry, and Seafood on the Rise [Internet]. Economic Research Service U.S. Department of Agriculture. 2 December 2019. Available online: https://www.ers.usda.gov/amber-waves/2019/december/us-per-capita-availability-of-red-meat-poultry-and-seafood-on-the-rise/ (accessed on 15 March 2022).
- Davis, C.G.; Lin, B.-H. Factors Affecting US Beef Consumption; US Department of Agriculture, Economic Research Service: Washington, DC, USA, 2005.
- Albracht-Schulte, K.; Islam, T.; Johnson, P.; Moustaid-Moussa, N. Systematic review of beef protein effects on gut microbiota: Implications for health. Adv. Nutr. 2021, 12, 102–114. [Google Scholar] [CrossRef]
- Page, J.; Wulf, D.; Schwotzer, T. A survey of beef muscle color and pH. J. Anim. Sci. 2001, 79, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, N.; Bach, A.; Velarde, A.; Devant, M. Association between animal, transportation, slaughterhouse practices, and meat pH in beef. Meat Sci. 2008, 78, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Padilla, I.; Leal-Escobar, G.; Garza-García, C.; Rodríguez-Castellanos, F. Dietary acid load: Mechanisms and evidence of its health repercussions. Nefrología 2019, 39, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, B.; Şimşek, A.; Claus, J.; Atılgan, E. Encapsulated phosphates reduce lipid oxidation in both ground chicken and ground beef during raw and cooked meat storage with some influence on color, pH, and cooking loss. Meat Sci. 2014, 97, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Eguchi, M.; Kurotani, K.; Kochi, T.; Pham, N.M.; Ito, R.; Kuwahara, K.; Tsuruoka, H.; Mizoue, T.; Kabe, I. High dietary acid load is associated with increased prevalence of hypertension: The Furukawa Nutrition and Health Study. Nutrition 2015, 31, 298–303. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Wallner, P.; Kundi, M.; Weisz, U.; Haas, W.; Hutter, H.P. Red meat, diseases, and healthy alternatives: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 247–261. [Google Scholar] [CrossRef]
- Papier, K.; Knuppel, A.; Syam, N.; Jebb, S.A.; Key, T.J. Meat consumption and risk of ischemic heart disease: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–12. [Google Scholar] [CrossRef]
- LeMieux, M.J.; Ramalingam, L.; Mynatt, R.L.; Kalupahana, N.S.; Kim, J.H.; Moustaïd-Moussa, N. Inactivation of adipose angiotensinogen reduces adipose tissue macrophages and increases metabolic activity. Obesity 2016, 24, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, M.; Pettitt, D.; Mott, D.; Knowler, W.; Nelson, R.; Bennett, P. Sequential changes in serum insulin concentration during development of non-insulin-dependent diabetes. Lancet 1989, 333, 1356–1359. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Al-Rubeaan, K.; Mohieldin, M.; Al-Katari, M.; Jones, A.F.; Kumar, S. Serum leptin and its relation to anthropometric measures of obesity in pre-diabetic Saudis. Cardiovasc. Diabetol. 2007, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, E.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Joosten, M.M.; Gans, R.O.; Navis, G.; Bakker, S.J. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 1811–1818. [Google Scholar] [CrossRef] [Green Version]
- Resnick, L.M.; Gupta, R.K.; Bhargava, K.K.; Gruenspan, H.; Alderman, M.H.; Laragh, J.H. Cellular ions in hypertension, diabetes, and obesity. A nuclear magnetic resonance spectroscopic study. Hypertension 1991, 17 Pt 2, 951–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robey, I.F. Examining the relationship between diet-induced acidosis and cancer. Nutr. Metab. 2012, 9, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinou-Tegou, A.; Kaloyianni, M.; Bourikas, D.; Koliakos, G. The effect of leptin on Na+-H+ antiport (NHE 1) activity of obese and normal subjects erythrocytes. Mol. Cell. Endocrinol. 2001, 183, 11–18. [Google Scholar] [CrossRef]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Beckles, A. Glucose intolerance following chronic metabolic acidosis in man. Am. J. Physiol.-Endocrinol. Metab. 1979, 236, E328. [Google Scholar] [CrossRef]
- Reaich, D.; Graham, K.A.; Channon, S.; Hetherington, C.; Scrimgeour, C.M.; Wilkinson, R.; Goodship, T. Insulin-mediated changes in PD and glucose uptake after correction of acidosis in humans with CRF. Am. J. Physiol.-Endocrinol. Metab. 1995, 268, E121–E126. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J.; Hu, F.B. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002, 25, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Guo, Y.; Bennett, D.A.; Bragg, F.; Bian, Z.; Chadni, M.; Yu, C.; Chen, Y.; Tan, Y.; Millwood, I.Y. Red meat, poultry and fish consumption and risk of diabetes: A 9 year prospective cohort study of the China Kadoorie Biobank. Diabetologia 2020, 63, 767–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Fu, J.; Moore, J.B.; Stoner, L.; Li, R. Processed and Unprocessed Red Meat Consumption and Risk for Type 2 Diabetes Mellitus: An Updated Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2021, 18, 10788. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, C. Salt intake, hypertension and diabetes mellitus. J. Hum. Hypertens. 2002, 16, S48–S51. [Google Scholar] [CrossRef] [Green Version]
- Cahill, L.E.; Pan, A.; Chiuve, S.E.; Sun, Q.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Fried-food consumption and risk of type 2 diabetes and coronary artery disease: A prospective study in 2 cohorts of US women and men. Am. J. Clin. Nutr. 2014, 100, 667–675. [Google Scholar] [CrossRef]
- Qin, P.; Liu, D.; Wu, X.; Zeng, Y.; Sun, X.; Zhang, Y.; Li, Y.; Wu, Y.; Han, M.; Qie, R. Fried-food consumption and risk of overweight/obesity, type 2 diabetes mellitus, and hypertension in adults: A meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–12. [Google Scholar] [CrossRef]
- Gadiraju, T.V.; Patel, Y.; Gaziano, J.M.; Djoussé, L. Fried food consumption and cardiovascular health: A review of current evidence. Nutrients 2015, 7, 8424–8430. [Google Scholar] [CrossRef]
- Honerlaw, J.P.; Ho, Y.-L.; Nguyen, X.-M.T.; Cho, K.; Vassy, J.L.; Gagnon, D.R.; O’Donnell, C.J.; Gaziano, J.M.; Wilson, P.W.; Djousse, L. Fried food consumption and risk of coronary artery disease: The million veteran program. Clin. Nutr. 2020, 39, 1203–1208. [Google Scholar] [CrossRef]
- Okuyama, H.; Langsjoen, P.H.; Ohara, N.; Hashimoto, Y.; Hamazaki, T.; Yoshida, S.; Kobayashi, T.; Langsjoen, A.M. Medicines and vegetable oils as hidden causes of cardiovascular disease and diabetes. Pharmacology 2016, 98, 134–170. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A. Abdominal obesity and type 2 diabetes in Asian Indians: Dietary strategies including edible oils, cooking practices and sugar intake. Eur. J. Clin. Nutr. 2017, 71, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Saeed, A.; Jordan, K.; Hoover, H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Rietman, A.; Schwarz, J.; Tomé, D.; Kok, F.J.; Mensink, M. High dietary protein intake, reducing or eliciting insulin resistance? Eur. J. Clin. Nutr. 2014, 68, 973–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marliss, E.B.; Vranic, M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: Implications for diabetes. Diabetes 2002, 51 (Suppl. 1), S271–S283. [Google Scholar] [CrossRef] [Green Version]
- Crouch, M.J.; Kosaraju, R.; Guesdon, W.; Armstrong, M.; Reisdorph, N.; Jain, R.; Fenton, J.; Shaikh, S.R. Frontline Science: A reduction in DHA-derived mediators in male obesity contributes toward defects in select B cell subsets and circulating antibody. J. Leukoc. Biol. 2019, 106, 241–257. [Google Scholar] [CrossRef]
- Ramalingam, L.; Menikdiwela, K.R.; Spainhour, S.; Eboh, T.; Moustaid-Moussa, N. Sex differences in early programming by maternal high fat diet induced-obesity and fish oil supplementation in mice. Nutrients 2021, 13, 3703. [Google Scholar] [CrossRef]






| Main Effects | Interactions | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Statistic | Protein (P) | Diet (D) | pH (H) | P × D | P × H | D × H | P × D × H |
| Final Body Weight | p | 0.3106 | <0.0001 | 0.1222 | 0.0754 | 0.4275 | 0.339 | 0.3473 |
| F (1, 67) | 1.044 | 90.27 | 2.45 | 3.261 | 0.6374 | 0.9274 | 0.3473 | |
| Fat Mass | p | 0.0058 | <0.0001 | 0.0312 | 0.1929 | 0.3824 | 0.351 | 0.5234 |
| F (1, 39) | 8.517 | 55.64 | 4.996 | 1.755 | 0.7806 | 0.8911 | 0.4145 | |
| Lean Mass | p | 0.0107 | 0.0397 | 0.1315 | 0.1045 | 0.3682 | 0.0287 | 0.2143 |
| F (1, 41) | 7.151 | 4.512 | 2.368 | 2.756 | 0.8279 | 5.14 | 1.591 | |
| Glucose Tolerance | p | 0.3203 | <0.0001 | 0.0303 | 0.5068 | 0.1034 | 0.1887 | 0.0427 |
| F (1, 67) | 1.003 | 77.27 | 4.896 | 0.4454 | 2.725 | 1.764 | 4.27 | |
| Serum Insulin | p | 0.4076 | <0.0001 | 0.2626 | 0.0198 | 0.1234 | 0.2888 | 0.4227 |
| F (1, 62) | 0.6951 | 57.37 | 1.278 | 5.721 | 2.439 | 1.145 | 0.6515 | |
| Serum Leptin | p | 0.6695 | <0.0001 | 0.1244 | 0.0678 | 0.3673 | 0.0544 | 0.257 |
| F (1, 67) | 0.1838 | 35.85 | 2.422 | 3.446 | 0.824 | 3.832 | 1.307 | |
| Main Effects | Interactions | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Statistic | Protein (P) | Diet (D) | pH (H) | P × D | P × H | D × H | P × D × H |
| Final Body Weight | p | 0.6346 | 0.0362 | 0.0296 | 0.0101 | 0.3357 | 0.4105 | 0.0412 |
| F (1, 64) | 0.228 | 4.578 | 4.952 | 7.027 | 0.9408 | 0.6862 | 4.342 | |
| Fat Mass | p | 0.709 | 0.0003 | 0.0574 | 0.0152 | 0.0767 | 0.0286 | 0.1438 |
| F (1, 65) | 0.1405 | 14.96 | 3.743 | 6.219 | 3.236 | 5.014 | 2.19 | |
| Lean Mass | p | 0.4621 | 0.2344 | 0.913 | 0.1049 | 0.9447 | 0.4794 | 0.9316 |
| F (1, 72) | 0.5466 | 1.438 | 0.01202 | 2.698 | 0.00484 | 0.5055 | 0.007409 | |
| Glucose Tolerance | p | 0.6806 | <0.0001 | 0.0058 | 0.1644 | 0.2628 | 0.0001 | 0.9436 |
| F (1, 67) | 0.1709 | 71.96 | 8.123 | 1.976 | 1.275 | 17.01 | 0.005036 | |
| Serum Insulin | p | 0.0578 | 0.2596 | 0.7709 | 0.0333 | 0.2827 | 0.0471 | 0.9625 |
| F (1, 68) | 3.725 | 1.292 | 0.08547 | 4.719 | 1.173 | 4.088 | 0.002224 | |
| Serum Leptin | p | 0.3825 | 0.0027 | 0.1099 | 0.098 | 0.9244 | 0.5423 | 0.4474 |
| F (1, 67) | 0.7728 | 9.683 | 2.625 | 2.816 | 0.009082 | 0.3751 | 0.5841 | |
| Main Effects | Interactions | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Statistic | Sex (S) | pH (H) | Protein (P) | S × H | S × P | H × P | S × H × P |
| Final Body Weight | p | <0.0001 | 0.2188 | 0.0001 | 0.8231 | 0.4582 | 0.0436 | 0.3772 |
| F (1, 66) | 213.6 | 1.541 | 16.23 | 0.05036 | 0.5569 | 4.235 | 0.7905 | |
| Fat Mass | p | 0.0006 | 0.3307 | <0.0001 | 0.2224 | 0.0173 | 0.9419 | 0.7368 |
| F (1, 59) | 13.33 | 0.9619 | 23.29 | 1.521 | 5.999 | 0.005365 | 0.1141 | |
| Lean Mass | p | <0.0001 | 0.9177 | <0.0001 | 0.3439 | 0.0603 | 0.7381 | 0.8619 |
| F (1, 62) | 491.7 | 0.01078 | 19.19 | 0.9097 | 3.662 | 0.1128 | 0.0305 | |
| Glucose Tolerance | p | <0.0001 | 0.995 | 0.7938 | 0.2754 | 0.0727 | 0.0071 | 0.0659 |
| F (1, 67) | 101.6 | 0.00003902 | 0.06887 | 1.21 | 3.326 | 7.716 | 3.495 | |
| Serum Insulin | p | <0.0001 | 0.4374 | 0.0015 | 0.3791 | 0.5267 | 0.2368 | 0.917 |
| F (1, 66) | 21.83 | 0.6106 | 11.01 | 0.7842 | 0.405 | 1.425 | 0.01095 | |
| Serum Leptin | p | <0.0001 | 0.8863 | 0.0756 | 0.5718 | 0.4699 | 0.7277 | 0.9462 |
| F (1, 67) | 43.3 | 0.02059 | 3.256 | 0.3229 | 0.5282 | 0.1223 | 0.00459 | |
| Main Effects | Interactions | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Statistic | Sex (S) | pH (H) | Protein (P) | S × H | S × P | H × P | S × H × P |
| Final Body Weight | p | <0.0001 | 0.0366 | 0.307 | 0.7619 | 0.7856 | 0.3275 | 0.4019 |
| F (1, 65) | 263.4 | 4.557 | 1.06 | 0.09261 | 0.07461 | 0.9734 | 0.7119 | |
| Fat Mass | p | <0.0001 | 0.0036 | 0.7379 | 0.4955 | 0.1287 | 0.9732 | 0.0723 |
| F (1, 45) | 83.35 | 9.442 | 0.1134 | 0.4721 | 2.396 | 0.001142 | 3.386 | |
| Lean Mass | p | <0.0001 | 0.1127 | 0.8603 | 0.0482 | 0.4317 | 0.3017 | 0.3069 |
| F (1, 51) | 304.2 | 2.605 | 0.03127 | 4.097 | 0.6281 | 1.089 | 1.065 | |
| Glucose Tolerance | p | <0.0001 | <0.0001 | 0.5796 | 0.4963 | 0.8495 | 0.8235 | 0.499 |
| F (1, 67) | 146.3 | 20.86 | 0.3099 | 0.4678 | 0.03628 | 0.05016 | 0.462 | |
| Serum Insulin | p | <0.0001 | 0.0234 | 0.028 | 0.3719 | 0.0397 | 0.0473 | 0.1708 |
| F (1, 63) | 154.1 | 5.402 | 5.061 | 0.8088 | 4.412 | 4.093 | 1.92 | |
| Serum Leptin | p | <0.0001 | 0.0216 | 0.1547 | 0.0874 | 0.2274 | 0.1942 | 0.2867 |
| F (1, 67) | 108.6 | 5.532 | 2.072 | 3.009 | 1.484 | 1.72 | 1.153 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menikdiwela, K.R.; Guimarães, J.P.T.; Scoggin, S.; Gollahon, L.S.; Moustaid-Moussa, N. Dietary pH Enhancement Improves Metabolic Outcomes in Diet-Induced Obese Male and Female Mice: Effects of Beef vs. Casein Proteins. Nutrients 2022, 14, 2583. https://doi.org/10.3390/nu14132583
Menikdiwela KR, Guimarães JPT, Scoggin S, Gollahon LS, Moustaid-Moussa N. Dietary pH Enhancement Improves Metabolic Outcomes in Diet-Induced Obese Male and Female Mice: Effects of Beef vs. Casein Proteins. Nutrients. 2022; 14(13):2583. https://doi.org/10.3390/nu14132583
Chicago/Turabian StyleMenikdiwela, Kalhara R., João Pedro Tôrres Guimarães, Shane Scoggin, Lauren S. Gollahon, and Naima Moustaid-Moussa. 2022. "Dietary pH Enhancement Improves Metabolic Outcomes in Diet-Induced Obese Male and Female Mice: Effects of Beef vs. Casein Proteins" Nutrients 14, no. 13: 2583. https://doi.org/10.3390/nu14132583