Exploring the Barriers and Motivators to Dietary Adherence among Caregivers of Children with Disorders of Amino Acid Metabolism (AAMDs): A Qualitative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical Framework
2.2. Participants’ Selection and Recruitments
2.3. Study Settings and Data Collection Procedures
2.4. Data Coding and Analysis
2.5. Trustworthiness
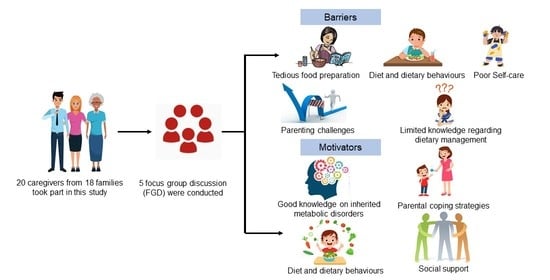
3. Results
3.1. Sociodemographic Characteristics
3.2. Barriers to Dietary Treatment
3.2.1. Burden of Dietary Treatment
Tedious Food Preparation
Difficulty Faced When Eating Out
Limited Food Portion
Limited Food Choices
Nutritional Value Contradiction
Challenges during Sick Day
3.2.2. Diet and Dietary Behavior
Food Choice
Eating Behavior
3.2.3. Parenting Challenges
Social Stress
Parental Stress
Poor Self-Control
Poor Self-Care Ability
3.2.4. Limited Knowledge Regarding Dietary Management
Uncertainty about Food Protein Content
Limited Food Preparation Knowledge
Uncertainty about Sick-Day Regime
3.2.5. Challenges Associated with Healthcare System Delivery
3.3. Motivators of Dietary Treatment
3.3.1. Good Knowledge of Inherited Metabolic Disorders
Good Knowledge of Dietary Treatment
Creativity in Meal Preparation
Health Concerns
3.3.2. Parental Coping Strategies
Moderation and Flexibility
Self-Prepared Food when Eating away from Home
Nutrition Education
Effective Self-Care
3.3.3. Social Support
The Healthcare System
Schools
Family Members
Subtheme 4: Other Caregivers
3.3.4. Diet and Dietary Behavior
Non-Picky Food Consumption
A Consistently Good Appetite
Eating Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezgu, F. Inborn Errors of Metabolism. Adv. Clin. Chem. 2016, 73, 195–250. [Google Scholar] [CrossRef]
- Shafie, A.A.; Supian, A.; Ahmad Hassali, M.A.; Ngu, L.H.; Thong, M.K.; Ayob, H.; Chaiyakunapruk, N. Rare disease in Malaysia: Challenges and solutions. PLoS ONE 2020, 15, e0230850. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.-O.; Rahman, S.A.-O.X.; Keller, M.A.-O.; Zschocke, J.A.-O. An international classification of inherited metabolic disorders (ICIMD). J. Inherit. Metab. Dis. 2021, 44, 164–177. [Google Scholar] [CrossRef]
- Waters, D.; Adeloye, D.; Woolham, D.; Wastnedge, E.; Patel, S.; Rudan, I. Global birth prevalence and mortality from inborn errors of metabolism: A systematic analysis of the evidence. J. Glob. Health 2018, 8, 021102. [Google Scholar] [CrossRef]
- Yunus, Z.M.; Kamaludin, D.A.; Mamat, M.; Choy, Y.S.; Ngu, L. Clinical and biochemical profiles of maple syrup urine disease in malaysian children. JIMD Rep. 2012, 5, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handoom, B.; Megdad, E.; Al-Qasabi, D.; Al Mesned, M.; Hawary, R.; Al-Nufiee, S.; Al-Hassnan, Z.; Alsayed, M.D.; Eldali, A. The effects of low protein products availability on growth parameters and metabolic control in selected amino acid metabolism disorders patients. Int. J. Pediatr. Adolesc. Med. 2018, 5, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Amit, N.; Ali, N.M.; Leong, H.Y.; Mohamad, M.; Rajikan, R. Effect of nutritional intervention on nutritional status among children with disorders of amino acid and nitrogen metabolism (AANMDs): A scoping review. Intractable Rare Dis. Res. 2021, 10, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Evans, S.; Pinto, A.; Ashmore, C.; Rocha, J.C.; MacDonald, A. A 3 Year Longitudinal Prospective Review Examining the Dietary Profile and Contribution Made by Special Low Protein Foods to Energy and Macronutrient Intake in Children with Phenylketonuria. Nutrients 2020, 12, 3153. [Google Scholar] [CrossRef]
- Boyer, S.W.; Barclay, L.J.; Burrage, L.C. Inherited Metabolic Disorders: Aspects of Chronic Nutrition Management. Nutr. Clin. Pract. 2015, 30, 502–510. [Google Scholar] [CrossRef] [Green Version]
- MaCdonald, A.; van Rijn, M.; Feillet, F.; Lund, A.M.; Bernstein, L.; Bosch, A.M.; Gizewska, M.; van Spronsen, F.J. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann. Nutr. Metab. 2012, 61, 289–295. [Google Scholar] [CrossRef] [PubMed]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Belanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Gizewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häberle, J.A.-O.X.; Burlina, A.; Chakrapani, A.; Dixon, M.; Karall, D.; Lindner, M.; Mandel, H.; Martinelli, D.; Pintos-Morell, G.; Santer, R.; et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J. Inherit. Metab. Dis. 2019, 42, 1192–1230. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.; MacDonald, A.; White, F.; Stafford, J. Disorders. In Clinical Paediatric Dietetics: Fourth Edition; John Wiley & Sons Ltd.: London, UK, 2014; pp. 381–525. [Google Scholar] [CrossRef]
- Garcia, M.I.; Araya, G.; Coo, S.; Waisbren, S.E.; de la Parra, A. Treatment adherence during childhood in individuals with phenylketonuria: Early signs of treatment discontinuation. Mol. Genet. Metab. Rep. 2017, 11, 54–58. [Google Scholar] [CrossRef]
- Stepien, K.M.; Geberhiwot, T.; Hendriksz, C.J.; Treacy, E.P. Challenges in diagnosing and managing adult patients with urea cycle disorders. J. Inherit. Metab. Dis. 2019, 42, 1136–1146. [Google Scholar] [CrossRef]
- Jurecki, E.R.; Cederbaum, S.; Kopesky, J.; Perry, K.; Rohr, F.; Sanchez-Valle, A.; Viau, K.S.; Sheinin, M.Y.; Cohen-Pfeffer, J.L. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017, 120, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.J.; Gutierrez, A.P.; Gassio, R.; Fuste, M.E.; Vilaseca, M.A.; Campistol, J. Neurological complications and behavioral problems in patients with phenylketonuria in a follow-up unit. Mol. Genet. Metab. 2011, 104, S73–S79. [Google Scholar] [CrossRef]
- Dababneh, S.; Alsbou, M.; Taani, N.; Sharkas, G.; Ismael, R.; Maraqa, L.; Nemri, O.; Al-Jawaldeh, H.; Kopti, N.; Atieh, E.; et al. Epidemiology of Phenylketonuria Disease in Jordan: Medical and Nutritional Challenges. Children 2022, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.I.; Cusmano-Ozog, K.; Enns, G.M.; Cowan, T.M. Correction of hyperleucinemia in MSUD patients on leucine-free dietary therapy. Mol. Genet. Metab. 2017, 122, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, S.; Wilson, B.J.; Graham, I.D.; Lamoureux, M.; Khangura, S.D.; Tingley, K.; Tessier, L.; Chakraborty, P.; Coyle, D.; Dyack, S.; et al. Experiences of caregivers of children with inherited metabolic diseases: A qualitative study. Orphanet J. Rare Dis. 2016, 11, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilginsoy, C.; Waitzman, N.; Leonard, C.O.; Ernst, S.L. Living with phenylketonuria: Perspectives of patients and their families. J. Inherit. Metab. Dis. 2005, 28, 639–649. [Google Scholar] [CrossRef]
- Kemper, A.R.; Brewer, C.A.; Singh, R.H. Perspectives on dietary adherence among women with inborn errors of metabolism. J. Am. Diet. Assoc. 2010, 110, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Sharman, R.; Mulgrew, K.; Katsikitis, M. Qualitative analysis of factors affecting adherence to the phenylketonuria diet in adolescents. Clin. Nurse Spec. 2013, 27, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Moreschi, C.; Toscano, A.; Comber, P.; Vegni, E. The PKU & ME study: A qualitative exploration, through co-creative sessions, of attitudes and experience of the disease among adults with phenylketonuria in Italy. Mol. Genet. Metab. Rep. 2020, 23, 100585. [Google Scholar] [CrossRef] [PubMed]
- Jarab, A.S.; Mukattash, T.L.; Al-Azayzih, A.; Khdour, M. A focus group study of patient’s perspective and experiences of type 2 diabetes and its management in Jordan. Saudi Pharm. J. 2018, 26, 301–305. [Google Scholar] [CrossRef]
- Wong, L.P. Focus group discussion: A tool for health and medical research. Singapore Med. J. 2008, 49, 256–261. [Google Scholar] [PubMed]
- Cigdem Sari, O.; Anibh Martin, D. Living with Glutaric Aciduria Type 1: Experiences of Adolescents and Their Families Living in Germany. Gazi Med. J. 2021, 32, 69–75. [Google Scholar]
- Alase, A. The Interpretative Phenomenological Analysis (IPA): A Guide to a Good Qualitative Research Approach. Lit. Numer. Stud. 2017, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm. Policy Ment. Health 2015, 42, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Guetterman, T.C. Descriptions of Sampling Practices within Five Approaches to Qualitative Research in Education and the Health Sciences. Forum Qual. Soz. Forum Qual. Soc. Res. 2015, 16. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef] [Green Version]
- Gale, N.K.; Heath, G.; Elaine, C.; Sabina, R.; Sab, I.R. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Fereday, J.; Muir-Cochrane, E. Demonstrating Rigor Using Thematic Analysis: A Hybrid Approach of Inductive and Deductive Coding and Theme Development. Int. J. Qual. Methods 2006, 5, 80–92. [Google Scholar] [CrossRef]
- Cloutier, C.; Ravasi, D. Using tables to enhance trustworthiness in qualitative research. Strateg. Organ. 2020, 19, 113–133. [Google Scholar] [CrossRef]
- Hadi, M.A.; Jose Closs, S. Ensuring rigour and trustworthiness of qualitative research in clinical pharmacy. Int. J. Clin. Pharm. 2016, 38, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Guest, G.; Namey, E.; McKenna, K. How Many Focus Groups Are Enough? Building an Evidence Base for Nonprobability Sample Sizes. Field Methods 2016, 29, 3–22. [Google Scholar] [CrossRef]
- Marijn Stok, F.; Renner, B.; Allan, J.; Boeing, H.; Ensenauer, R.; Issanchou, S.; Kiesswetter, E.; Lien, N.; Mazzocchi, M.; Monsivais, P.; et al. Dietary Behavior: An Interdisciplinary Conceptual Analysis and Taxonomy. Front. Psychol. 2018, 9, 1689. [Google Scholar] [CrossRef] [Green Version]
- Bösch, F.; Landolt, M.A.; Baumgartner, M.R.; Zeltner, N.; Kölker, S.; Gleich, F.; Burlina, A.; Cazzorla, C.; Packman, W.; VDSchwartz, I.; et al. Health-related quality of life in paediatric patients with intoxication-type inborn errors of metabolism: Analysis of an international data set. J. Inherit. Metab. Dis. 2021, 44, 215–225. [Google Scholar] [CrossRef]
- Campbell, H.; Singh, R.H.; Hall, E.; Ali, N. Caregiver Quality of Life with Tyrosinemia Type 1. J. Genet. Couns. 2018, 27, 723–731. [Google Scholar] [CrossRef]
- Morawska, A.; Mitchell, A.E.; Etel, E.; Kirby, G.; McGill, J.; Coman, D.; Inwood, A. Psychosocial functioning in children with phenylketonuria: Relationships between quality of life and parenting indicators. Child Care Health Dev. 2020, 46, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Baumstarck, K.; Cano, A.; Loundou, A.; Berbis, J.; Chabrol, B.; Auquier, P.; Chesney, M.A.; Neilands, T.B.; Chambers, D.B.; et al. Assessment of quality of life of the children and parents affected by inborn errors of metabolism with restricted diet: Preliminary results of a cross-sectional study. Health Qual. Life Outcomes 2013, 11, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, B.; Schwahn, B.; Galloway, P.; Robinson, P.; Gerasimidis, K. A questionnaire survey on the usage of low protein staple foods by people with phenylketonuria in Scotland. J. Hum. Nutr. Diet 2014, 27, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Almeida, M.F.; van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Gokmen-Ozel, H.; Lammardo, A.M.; MacDonald, A.; Robert, M.; et al. Special low protein foods for phenylketonuria: Availability in Europe and an examination of their nutritional profile. Orphanet J. Rare Dis. 2015, 10, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, G.; Pinto, A.; Evans, S.; Daly, A.; Adams, S.; Costelloe, S.; Gribben, J.; Ellerton, C.; Emm, A.; Firman, S.; et al. Special Low Protein Foods Prescribed in England for PKU Patients: An Analysis of Prescribing Patterns and Cost. Nutrients 2021, 13, 3977. [Google Scholar] [CrossRef] [PubMed]
- Mlcoch, T.; Puda, R.; Jesina, P.; Lhotakova, M.; Sterbova, S.; Dolezal, T. Dietary patterns, cost and compliance with low-protein diet of phenylketonuria and other inherited metabolic diseases. Eur. J. Clin. Nutr. 2018, 72, 87–92. [Google Scholar] [CrossRef]
- Trefz, F.; Muntau, A.C.; Schneider, K.M.; Altevers, J.; Jacob, C.; Braun, S.; Greiner, W.; Jha, A.; Jain, M.; Alvarez, I.; et al. Health economic burden of patients with phenylketonuria (PKU)—A retrospective study of German health insurance claims data. Mol. Genet. Metab. Rep. 2021, 27, 100764. [Google Scholar] [CrossRef]
- Poole, G.; Pinto, A.; Evans, S.; Ford, S.; O’Driscoll, M.; Buckley, S.; Ashmore, C.; Daly, A.; MacDonald, A. Hungry for Change: The Experiences of People with PKU, and Their Caregivers, When Eating Out. Nutrients 2022, 14, 626. [Google Scholar] [CrossRef]
- Medford, E.; Hare, D.J.; Wittkowski, A. Demographic and Psychosocial Influences on Treatment Adherence for Children and Adolescents with PKU: A Systematic Review. JIMD Rep. 2018, 39, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Witalis, E.; Mikoluc, B.; Motkowski, R.; Sawicka-Powierza, J.; Chrobot, A.; Didycz, B.; Lange, A.; Mozrzymas, R.; Milanowski, A.; Nowacka, M.; et al. Phenylketonuria patients’ and their parents’ knowledge and attitudes to the daily diet—Multi-centre study. Nutr. Metab. 2017, 14, 57. [Google Scholar] [CrossRef]
- Packman, W.; Mehta, I.; Rafie, S.; Mehta, J.; Naldi, M.; Mooney, K.H. Young adults with MSUD and their transition to adulthood: Psychosocial issues. J. Genet. Couns. 2012, 21, 692–703. [Google Scholar] [CrossRef]
- Di Ciommo, V.; Forcella, E.; Cotugno, G. Living With Phenylketonuria From the Point of View of Children, Adolescents, and Young Adults: A Qualitative Study. J. Dev. Behav. Pediatrics 2012, 33, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cazzorla, C.; Bensi, G.; Biasucci, G.; Leuzzi, V.; Manti, F.; Musumeci, A.; Papadia, F.; Stoppioni, V.; Tummolo, A.; Vendemiale, M.; et al. Living with phenylketonuria in adulthood: The PKU ATTITUDE study. Mol. Genet. Metab. Rep. 2018, 16, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Opiyo, R.O.; Nyasulu, P.S.; Olenja, J.; Zunza, M.; Nguyen, K.A.; Bukania, Z.; Nabakwe, E.; Mbogo, A.; Were, A.O. Factors associated with adherence to dietary prescription among adult patients with chronic kidney disease on hemodialysis in national referral hospitals in Kenya: A mixed-methods survey. Ren. Replace Ther. 2019, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Haitjema, S.; Lubout, C.M.A.; Abeln, D.; Bruijn-van der Veen, M.; MacDonald, A.; Wolffenbuttel, B.H.R.; van Spronsen, F.J. Dietary treatment in Dutch children with phenylketonuria: An inventory of associated social restrictions and eating problems. Nutrition 2021, 97, 111576. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Alroqaiba, N.; Daly, A.; Neville, C.; Davies, P.; Macdonald, A. Feeding difficulties in children with inherited metabolic disorders: A pilot study. J. Hum. Nutr. Diet. 2012, 25, 209–216. [Google Scholar] [CrossRef]
- Andrew, M.J.; Parr, J.R.; Sullivan, P.B. Feeding difficulties in children with cerebral palsy. Arch. Dis. Child. Educ. Pract. Ed. 2012, 97, 222. [Google Scholar] [CrossRef] [PubMed]
- Ozel, H.G.; Kucukkasap, T.; Koksal, G.; Sivri, H.S.; Dursun, A.; Tokatli, A.; Coskun, T. Does maternal knowledge impact blood phenylalanine concentration in Turkish children with phenylketonuria? J. Inherit. Metab. Dis. 2008, 31 (Suppl. 2), S213–S217. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Davies, P.; Daly, A.; Hopkins, V.; Hall, S.K.; Asplin, D.; Hendriksz, C.; Chakrapani, A. Does maternal knowledge and parent education affect blood phenylalanine control in phenylketonuria? J. Hum. Nutr. Diet 2008, 21, 351–358. [Google Scholar] [CrossRef]
- De Castro, M.; Turner, C.; Kirmse, B. Practical recommendations for the transition to adulthood for the adolescent with a genetic diagnosis. Special emphasis on inborn errors of metabolism. Transl. Sci. Rare Dis. 2019, 2019, 159–168. [Google Scholar] [CrossRef] [Green Version]

| General Questions | Probing Questions |
|---|---|
| 1. Can you share your experience in managing your child’s daily diet? | 1. What are the barriers that you faced when managing your child’s daily diet in term of low protein diet and metabolic formula? 2. What are the situations that always trigger non-adherence to dietary treatment? 3. How do you overcome the challenges associated with dietary treatment? 4. What are the factors that continue motivating you to practice the low protein diet although it’s challenging? |
| Characteristics (n = 20) | n (%) |
|---|---|
| Gender Male Female | 2 (10%) 18 (90%) |
| Age of caregiver 31–40 41–50 >50 | 10 (50%) 9 (45%) 1 (5%) |
| Age of patients (n = 24) 0–3 4–6 7–9 10–12 13–15 16–18 | 5 (20.8%) 5 (20.8%) 4 (16.7%) 3 (12.5%) 4 (16.7%) 3 (12.5%) |
| Races Malay Chinese Indian | 13 (65%) 6 (30%) 1 (5%) |
| Educational level Secondary school Diploma/degree | 8 (40%) 12 (60%) |
| Employment Currently working Housewife Retirees | 11 (55%) 8 (40%) 1 (5%) |
| Types of AAMDs of children (n = 18) Aminoacidopathies (AAs) Urea cycle disorders (UCDs) Organic acidurias (OAs) | 7 (38.8%) 5 (27.8%) 6 (33.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.Y.; Rajikan, R.; Amit, N.; Ali, N.M.; Hamid, H.A.; Leong, H.Y.; Mohamad, M.; Koh, B.Q.; Musa, A. Exploring the Barriers and Motivators to Dietary Adherence among Caregivers of Children with Disorders of Amino Acid Metabolism (AAMDs): A Qualitative Study. Nutrients 2022, 14, 2535. https://doi.org/10.3390/nu14122535
Lim JY, Rajikan R, Amit N, Ali NM, Hamid HA, Leong HY, Mohamad M, Koh BQ, Musa A. Exploring the Barriers and Motivators to Dietary Adherence among Caregivers of Children with Disorders of Amino Acid Metabolism (AAMDs): A Qualitative Study. Nutrients. 2022; 14(12):2535. https://doi.org/10.3390/nu14122535
Chicago/Turabian StyleLim, Jing Ying, Roslee Rajikan, Noh Amit, Nazlena Mohamad Ali, Haslina Abdul Hamid, Huey Yin Leong, Maslina Mohamad, Bi Qi Koh, and Aini Musa. 2022. "Exploring the Barriers and Motivators to Dietary Adherence among Caregivers of Children with Disorders of Amino Acid Metabolism (AAMDs): A Qualitative Study" Nutrients 14, no. 12: 2535. https://doi.org/10.3390/nu14122535