Pressurized Liquid Extraction Combined with Enzymatic-Assisted Extraction to Obtain Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace
Abstract
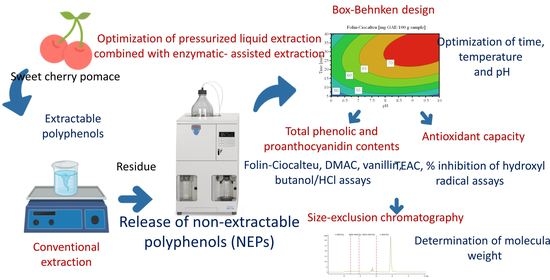
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Conventional Extraction of Extractable Polyphenols (EPPs)
2.4. Release of Non-Extractable Polyphenols (NEPs)
2.5. Total Phenolic Content (TPC)
2.6. Total Proanthocyanidin Content
2.6.1. DMAC Assay
2.6.2. Vanillin Assay
2.6.3. HCl/Butanol Assay
2.7. Antioxidant Capacity Determination
2.7.1. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.7.2. Capacity to Inhibit the Formation of Hydroxyl Radical Assay
2.8. High-Performance Liquid Size-Exclusion Chromatography (HPLC-SEC) Determination of Molecular Weight of NEPs from Sweet Cherry Pomace Extracts
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of NEPs Extraction from Cherry Pomace Extraction Residue by PLE Combined with EAE
3.2. Comparison of PLE Combined with EAE and PLE Alone to Release NEPs from Cherry Pomace Extraction Residue
3.3. Determination of Molecular Weight of NEPs by Size Exclusion Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Mehmet Yılmaz, F.; Mehmet, K.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef] [Green Version]
- Kolodziejezyk, K.; Sojka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crops Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Da Silva, D.B.; Giombelli, C.; Gallani, D.L.; Stevanato, N.; Da Silva, C.; Bolanho, B.C. Ultrasound-assisted extraction of phenolic compounds and soluble sugars from the stem portion of peach palm. J. Food Process. Preserv. 2020, 44, e14636. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of phenolic antioxidants extraction from Fucus vesiculosus by pressurized liquid extraction. J. Appl. Phycol. 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Ding, Y.; Morozova, K.; Scampicchio, M.; Ferrentino, G. Non-extractable polyphenols from food by-products: Current knowledge on recovery, characterization, and potential applications. Processes 2020, 8, 925. [Google Scholar] [CrossRef]
- Tow, W.W.; Premier, R.; Jing, H.; Ajlouni, S. Antioxidant and antiproliferation effects of extractable and nonextractable polyphenols isolated from apple waste using different extraction methods. J. Food Sci. 2011, 76, 163–172. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, Z.; Wang, K.; Song, S.; Shao, H.; Yang, X. Chemical profile and antioxidant potential of extractable and non-extractable polyphenols in commercial teas at different fermentation degrees. J. Food Process. Preserv. 2020, 44, e14487. [Google Scholar] [CrossRef]
- Arranz, S.; Saura Calixto, F. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant potential in nonextractable fractions of dried persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folinciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Miron, L.T.; Herrero, M.; Ibáñez, E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chromatogr. A 2013, 1288, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Camargo, A.P.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Condezo-Hoyos, L.; Mohanty, I.P.; Noratto, G.D. Assessing non-digestible compounds in apple cultivars and their potential as modulators of obese faecal microbiota in vitro. Food Chem. 2014, 161, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kosar, M.; Dorman, H.J.D.; Hiltunen, R. Effect of an acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae species. Food Chem. 2005, 91, 525–533. [Google Scholar] [CrossRef]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Profiling of phenolic compounds from different apple varieties using comprehensive two-dimensional liquid chromatography. J. Chromatogr. A 2013, 1313, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.F.; Li, C.M.; Xu, Y.; Hu, W.; Chen, M.; Wan, Q. Structural features and antioxidant activity of tannin from persimmon pulp. Food Res. Int. 2008, 41, 208–217. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Saura-Calixto, F. Proanthocyanidin content in foods is largely underestimated in the literature data: An approach to quantification of the missing proanthocyanidins. Food Res. Int. 2009, 42, 1381–1388. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 9–10. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluk, R.E. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int. J. Mol. 2011, 12, 6685–6702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinelo, M.; Meyer, S. Enzyme-assited extraction of antioxidants: Release of phenols from vegetal matrixes. Electron. J. Environ. Agric. Food Chem. 2008, 7, 3217–3220. [Google Scholar]
- Yahia, E.M.; Carrillo-López, A.; Bello-Perez, L.A. Carbohydrates, Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2019; Chapter 9; pp. 175–205. [Google Scholar]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef]
- Ramos, O.L.; Malcata, F.X. Food-Grade enzymes. Compr. Biotechnol. 2017, 3, 587–603. [Google Scholar] [CrossRef]
- Suberkropp, K. Pectin-degrading enzymes: Polygalacturonase and pectin lyase. In Methods to Study Litter Decomposition; Springer: Cham, Switzerland, 2020; pp. 419–424. [Google Scholar] [CrossRef]
- Schaich, K.M.; Xie, X.T.J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods. 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Monteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Opitz, S.E.; Smrke, S.; Goodman, B.A.; Yeretzian, C. Methodology for the measurement of antioxidant Capacity of coffee. In Processing and Impact on Antioxidants in Beverages; Academic Press: London, UK, 2014; pp. 253–264. [Google Scholar] [CrossRef]



| Parameters | Folin-Ciocalteu | p-Value | DMAC | p-Value | Vanillin | p-Value | Butanol/HCl | p-Value | TEAC | p-Value | Hydroxyl Radical | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constant | 76.5687 | 0.714975 | 50.4326 | 2.31323 | 0.00631004 | 12.5415 | ||||||
| t | 3.74087 | 0.0690312 | 0.0988549 | 0.0757302 | 1.48121 | 0.274243 | −1.55757 | 0.259711 | −0.00057974 | 0.023165 | −0.226677 | 0.874268 |
| T | −0.642173 | 0.708234 | −0.0983441 | 0.0768368 | −2.6666 | 0.0781056 | −1.7044 | 0.244879 | 0.0008498 | 0.0122791 | 3.93067 | 0.0248276 |
| p | 4.92316 | 0.0288109 | 0.0657091 | 0.197594 | −0.972796 | 0.432818 | −5.1847 | 0.0143429 | 0.00068073 | 0.0136543 | −7.98952 | 0.00067382 |
| t2 | −5.30567 | 0.0321596 | −0.12659 | 0.0499341 | −1.84528 | 0.176053 | 1.47379 | 0.383005 | 0.00066431 | 0.0270325 | −2.92402 | 0.0664017 |
| T2 | −4.74775 | 0.0463558 | −0.020252 | 0.697806 | 4.16827 | 0.0162499 | −0.925015 | 0.517185 | 0.00037737 | 0.0519364 | 4.04103 | 0.019886 |
| p2 | −3.79391 | 0.0892811 | 0.0230183 | 0.659731 | 7.39256 | 0.00278374 | 5.73637 | 0.0116925 | −0.00072299 | 0.0206362 | 9.85783 | 0.00015956 |
| t*T | −0.902904 | 0.624473 | −0.0188704 | 0.70636 | 0.305061 | 0.84502 | 0.874684 | 0.510832 | 9.47 × 10−5 | 0.653919 | −0.755402 | 0.601397 |
| t*p | 2.94867 | 0.149462 | −0.0406045 | 0.429809 | −0.903755 | 0.458469 | 3.93605 | 0.0315481 | 0.00025249 | 0.140774 | −2.08563 | 0.174755 |
| T*p | 0.182355 | 0.920256 | −0.0668348 | 0.216721 | 4.09835 | 0.0148862 | −1.64187 | 0.361947 | −8.93 × 10−5 | 0.672088 | −1.09081 | 0.455551 |
| R2 | 0.876 | 0.816 | 0.943 | 0.931 | 0.971 | 0.941 | ||||||
| RSD | 6.063 | 0.1655 | 4.232 | 4.237 | 0.000517 | 5.524 | ||||||
| p-value (test of regression) | 0.073 | 0.167 | 0.012 | 0.05 | 0.009 | 0.002 | ||||||
| p-value (lack of fit) | 0.65 | 0.346 | 0.946 | 0.109 | 0.213 | 0.492 | ||||||
| Optimal EAE Conditions | Theoretical Values | Experimental Values | |||
|---|---|---|---|---|---|
| Optimum Value | Lower | Upper | PLE with PromodTM Enzyme | PLE without Enzyme | |
| TPC (mg GAE/100 g sample) | 72.1 | 57.7 | 86.5 | 75 ± 8 a | 14 ± 1 b |
| DMAC (mg epicatechin/100 g sample) | 1.04 | 0.65 | 1.44 | 0.97 ± 0.07 a | 0.24 ± 0.03 b |
| Vanillin (mg epicatechin/100 g sample) | 66.2 | 53.8 | 78.6 | 76 ± 8 a | 30 ± 8 b |
| Butanol/HCl (mg epicatechin/100 g sample) | 13.2 | 2.4 | 24.2 | 20 ± 2 a | 11.6 ± 0.9 b |
| TEAC (µmol Trolox/g sample) | 0.0056 | 0.0039 | 0.0074 | 0.0051 ± 0.0006 a | 0.0027 ± 0.0008 b |
| Hydroxyl radical assay (% of hydroxyl radical inhibition) | 22.8 | 10.8 | 34.9 | 20 ± 4 a | 10 ± 2 b |
| Extraction Method | >8000 Da | 8000–6000 Da | 6000–2000 Da | <2000 Da |
|---|---|---|---|---|
| PLE with PromodTM enzyme | 2145 ± 70 | 1604 ± 183 | 1760 ± 38 | 8219 ± 49 |
| PLE without enzyme | 811 ± 400 | 1376 ± 223 | 569 ± 49 | 15,580 ± 284 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Rodríguez, G.; García, M.C.; Marina, M.L.; Plaza, M. Pressurized Liquid Extraction Combined with Enzymatic-Assisted Extraction to Obtain Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace. Nutrients 2021, 13, 3242. https://doi.org/10.3390/nu13093242
Domínguez-Rodríguez G, García MC, Marina ML, Plaza M. Pressurized Liquid Extraction Combined with Enzymatic-Assisted Extraction to Obtain Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace. Nutrients. 2021; 13(9):3242. https://doi.org/10.3390/nu13093242
Chicago/Turabian StyleDomínguez-Rodríguez, Gloria, María Concepción García, María Luisa Marina, and Merichel Plaza. 2021. "Pressurized Liquid Extraction Combined with Enzymatic-Assisted Extraction to Obtain Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace" Nutrients 13, no. 9: 3242. https://doi.org/10.3390/nu13093242