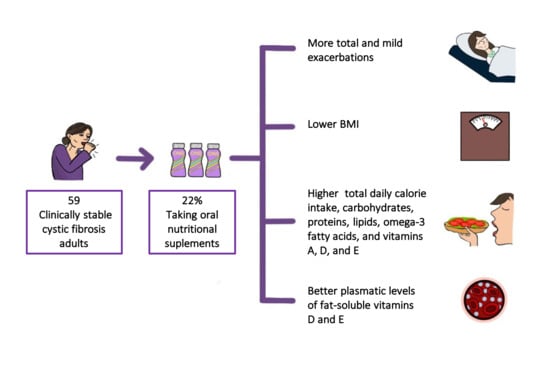
Oral Nutritional Supplements in Adults with Cystic Fibrosis: Effects on Intake, Levels of Fat-Soluble Vitamins, and Bone Remodeling Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anthropometric and Body Composition Parameters
2.2. Dietary Questionnaire
2.3. Laboratory Measurements
2.4. Assessment of Respiratory Status
2.5. Statistical Analyses
2.6. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Culhane, S.; George, C.; Pearo, B.; Spoede, E. Malnutrition in cystic fibrosis: A review. Nutr. Clin. Pract. 2013, 28, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 3, 557–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olveira, G.; Olveira, C. Nutrition, cystic fibrosis and the digestive tract. Nutr. Hosp. 2008, 23, 71–86. [Google Scholar]
- Gomes, A.; Hutcheon, D. Association between Fat-Free Mass and Pulmonary Function in Patients with Cystic Fibrosis: A Narrative Review. Nutr. Clin. Pract. 2019, 34, 715–727. [Google Scholar] [CrossRef]
- Calella, A.P.; Valerio, G.; Thomas, M.; Mccabe, H. Association between body composition and pulmonary function in children and. Nutrition 2018, 48, 73–76. [Google Scholar] [CrossRef]
- Smyth, R.L.; Rayner, O. Oral calorie supplements for cystic fibrosis. Cochrane Database Syst. Rev. 2017, 5, CD000406. [Google Scholar] [CrossRef] [Green Version]
- Woestenenk, J.W.; Castelijns, S.J.A.M.; Van der Ent, C.K.; Houwen, R.H.J. Dietary intake in children and adolescents with cystic fibrosis. Clin. Nutr. 2014, 33, 528–532. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Torralvo, F.J.S.; Porras, N.; Fernández, J.A.; Torres, F.G.; Tapia, M.J.; Lima, F.; Escofet, F.J.S.; Marín, M.G.; Martínez, G.R.; Olveira, G. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar]
- Olveira, G.; Olveira, C.; Casado-Miranda, E.; Padilla, A.; Dorado, A.; Rojo-Martinez, G.; Porras, N.; Garcia-Escobar, E.; Soriguer, F. Markers for the validation of reported dietary intake in adults with cystic fibrosis. J. Am. Diet. Assoc. 2009, 109, 1704–1711. [Google Scholar] [CrossRef]
- Jimenez, A. Tablas de Composición de Alimentos; Barcelona Novartis Consum Heal SA: Barcelona, Spain, 2002. [Google Scholar]
- Mataix, J. Tablas de Composición de Alimentos Españoles; Granada Univ Granada: Granada, Spain, 2003. [Google Scholar]
- Spanish Food Composition Database. Available online: https://www.bedca.net/bdpub/ (accessed on 2 January 2021).
- Soriguer, F.; Serna, S.; Valverde, E.; Hernando, J.; Soriguer, M.; Pareja, A.; Tinahones, F.; Esteva, I. Fat, protein and caloric content of different fish, seafood and mollusks, Atlantic and Mediterranean habitually consumed in the south of Spain. Nutr. Hosp. 1996, 11, 245–257. [Google Scholar] [PubMed]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/download-datasets.html (accessed on 22 November 2020).
- Máiz, L.; Baranda, F.; Coll, R.; Prados, C.; Vendrell, M.; Escribano, A.; Gartner, S.; de Gracia, S.; Martínez, M.; Salcedo, A.; et al. SEPAR (Spanish Society of Pneumology and Thoracic Surgery) Guidelines. Guideline for diagnosis and treatment of respiratory involvements in cystic fibrosis. Arch. Bronconeumol. 2001, 37, 316–324. [Google Scholar] [CrossRef]
- Roca, J.; Sanchis, J.; Segarra, A.A.V.F.; Navaias, D. Spirometric reference values from a Mediterranean population. Bull. Eur. Physiopathol. Respir. 1986, 22, 217–224. [Google Scholar]
- Hollander, F.M.; van Pierre, D.D.; de Roos, N.M.; van de Graaf, E.A.; Iestra, J.A. Effects of nutritional status and dietetic interventions on survival in Cystic Fibrosis patients before and after lung transplantation. J. Cyst. Fibros. 2014, 13, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Bolívar, V.; Olveira, C.; Blasco, J.; Olveira, G. Actualización en nutrición en la fibrosis quistica. Nutr. Clin. Med. 2019, 1, 19–44. [Google Scholar]
- Skypala, I.; Ashworth, F.; Hodson, M.; Leonard, C.; Knox, A.; Hiller, E.; Wolfe, S.; Littlewood, J.; Morton, A.; Conway, S.; et al. Oral nutritional supplements promote significant weight gain in cystic fibrosis patients. J. Hum. Nutr. Diet. 2008, 11, 95–104. [Google Scholar] [CrossRef]
- Poustie, V.J.; Russell, J.E.; Watling, R.M.; Ashby, D.; Smyth, R.L. Oral protein energy supplements for children with cystic fibrosis: CALICO multicentre randomised controlled trial. Bmj 2006, 332, 632–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappler, M.; Griese, M. Nutritional supplements in cystic fibrosis. Bmj. 2006, 332, 618–619. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.L.; Lee, C.K.K.; Mogayzel, P.J.; Zeitlin, P.L.; Rosenstein, B.J. Dietary supplement use in pediatric patients with cystic fibrosis. Am. J. Health Pharm. 2008, 65, 562–565. [Google Scholar] [CrossRef]
- Olveira, C.; Sole, A.; Girón, R.M.; Quintana-Gallego, E.; Mondejar, P.; Baranda, F.; Alvarez, A.; Prados, C.; Rodríguez-González, J.; Herrero-Labarga, I.; et al. Depression and anxiety symptoms in Spanish adult patients with cystic fibrosis: Associations with health-related quality of life. Gen. Hosp. Psychiatry 2016, 40, 39–46. [Google Scholar] [CrossRef]
- Delion, M.; Braux, J.; Jourdain, M.L.; Guillaume, C.; Bour, C.; Gangloff, S.; Pimpec-Barthes, F.L.; Sermet-Gaudelus, I.; Jacquot, J.; Velard, F. Overexpression of RANKL in osteoblasts: A possible mechanism of susceptibility to bone disease in cystic fibrosis. J. Pathol. 2016, 240, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Nieves, J.W. Osteoporosis: The role of micronutrients. Am. J. Clin. Nutr. 2005, 81, 1232S–1239S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, J.K.D.; Diaz, M.A.N.; Pizziolo, V.R.; Martino, H.S.D. Effect of vitamin K in bone metabolism and vascular calcification: A review of mechanisms of action and evidences. Crit. Rev. Food Sci. Nutr. 2017, 57, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Basset, G.J.; Latimer, S.; Fatihi, A.; Soubeyrand, E.; Block, A. Phylloquinone (Vitamin K1): Occurrence, Biosynthesis and Functions. Mini Rev. Med. Chem. 2016, 17, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.P. Vitamin K in cystic fibrosis. J. R. Soc. Med. 2004, 97, 48–51. [Google Scholar]
- Conway, S.P.; Wolfe, S.P.; Brownlee, K.G.; White, H.; Oldroyd, B.; Truscott, J.G.; Harvey, J.M.; Shearer, M.J. Vitamin K status among children with cystic fibrosis and its relationship to bone mineral density and bone turnover. Pediatrics 2005, 115, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
| CF | CF | ||||
|---|---|---|---|---|---|
| Total | No ONS (n = 46) | ONS (n = 13) | p | ||
| Age | (m ± SD) | 29.3 ± 9.4 | 29.8 ± 9.7 | 27.4 ± 8.2 | NS |
| Sex | NS | ||||
| Male Female | n (%) | 25 (42.4) 34 (57.6) | 17 (36.9) 29 (63) | 8 (61.5) 5 (38.4) | |
| Respiratory status | |||||
| Bronchorrhea (mL) | (m ± SD) | 21.3 ± 20.8 | 19.7 ± 19.2 | 25.8 ± 23.2 | NS |
| Total exacerbations | (m ± SD) | 2.3 ± 1.8 | 2.0 ± 1.5 | 3.6 ± 2.2 | <0.01 |
| Mild exacerbations | (m ± SD) | 1.9 ± 1.6 | 1.6 ± 1.3 | 3.2 ± 1.8 | <0.01 |
| Severe exacerbations | (m ± SD) | 0.41 ± 0.80 | 0.4 ± 0.8 | 0.5 ± 0.8 | NS |
| FEV1 (%) | (m ± SD) | 63.3 ± 25.6 | 66.9 ± 25.1 | 51.6 ± 24.9 | NS |
| FVC (%) | (m ± SD) | 75.3 ± 22.0 | 77.5 ± 22.3 | 66.9 ± 19.6 | NS |
| FEV1/FVC (%) | (m ± SD) | 66.7 ± 12.0 | 68.0 ± 15.7 | 61.4 ± 17.8 | NS |
| Colonisations | |||||
| Colonisation by S. Aureus | n (%) | 47 (79.7) | 35 (76.1) | 12 (92.3) | NS |
| Colonisation by H. influenzae | n (%) | 28 (47.5) | 19 (41.3) | 9 (69.2) | NS |
| Colonisation by P. aeruginosa | n (%) | 47 (79.7) | 36 (78.3) | 11 (84.6) | NS |
| CF | CF | ||||
|---|---|---|---|---|---|
| Total | No ONS (n = 46) | ONS (n = 13) | p | ||
| Bone status | |||||
| BMD (g/cm2) | (m ± SD) | 1.090 ± 0.2330 | 1.092 ± 0.250 | 1.086 ± 0.175 | NS |
| T-score | (m ± SD) | −0.532 ± 1.295 | −0.48 ± 1.25 | −0.76 ± 1.55 | NS |
| Z-score | (m ± SD) | −0.502 ± 1.185 | −0.46 ± 1.09 | −0.64 ± 1.49 | NS |
| Normal | n (%) | 39 (66.1) | 32 (69.5) | 7 (53.8) | NS |
| Osteopenia | n (%) | 15 (25.4) | 11 (23.9) | 4 (30.8) | |
| Osteoporosis | n (%) | 5 (8.5) | 3 (6.6) | 2 (15.4) | |
| Nutritional status | n = 40 | n = 12 | |||
| BMI | (m ± SD) | 22.0 ± 3.6 | 22.5 ±3.7 | 20.1 ±2.3 | <0.05 |
| Fat mass (kg) | (m ± SD) | 15.9 ± 8.5 | 16.8 ± 8.9 | 12.4 ± 6.1 | NS |
| Fat-free mass (kg) | (m ± SD) | 42.8 ± 9.4 | 43.2 ± 9.9 | 41.5 ±7.8 | NS |
| Fat mass (%) | (m ± SD) | 26.3 ± 10.7 | 27.3 ± 10.4 | 23.0 ± 11.6 | NS |
| Fat-free mass (%) | (m ± SD) | 73.7 ± 10.7 | 72.7 ±10.4 | 76.9 ±11.6 | NS |
| FFMI (kg/m2) | (m ± SD) | 16.3 ± 2.4 | 16.5 ± 2.4 | 15.6 ± 2.3 | NS |
| Malnourished (FFMI <17 M/15F) | n (%) | 29 | 20 (50) | 9 (75.3) | NS |
| Strength | n 46 | n 13 | |||
| Hand-grip dynamometry | (m ± SD) | 30.3 ± 7.3 | 30.4 ± 11.9 | 29.5 ± 9.5 | NS |
| CF | CF | ||||
|---|---|---|---|---|---|
| Total | No ONS (n = 46) | ONS (n = 13) | p | ||
| DIETARY QUESTIONNAIRE (n = 59) * | |||||
| Mean calorie intake | (m ± SD) | 2663.4 ± 574.3 | 2456.0 ± 497.3 | 2982.4 ± 553.3 | <0.01 |
| Calories/kg of body weight | (m ± SD) | 47.4 ± 11.7 | 41.3 ± 9.0 | 56.8 ± 8.9 | <0.001 |
| Calories from supplements (% of total) | (m ± SD) | - | 16.0 ± 10.4 | - | |
| Carbohydrates (%) | (m ± SD) | 44.7 ± 5.3 | 45.1 ± 6.0 | 44.0 ± 4.3 | NS |
| Carbohydrates/kg of body weight | (m ± SD) | 5.3 ± 1.4 | 4.7 ± 1.2 | 6.2 ± 1.1 | <0.01 |
| Proteins (%) | (m ± SD) | 16.1 ± 2.4 | 15.2 ± 2.2 | 17.3 ± 2.2 | <0.05 |
| Proteins/kg of body weight | (m ± SD) | 1.9 ± 0.6 | 1.6 ± 0.4 | 2.4 ± 0.4 | <0.001 |
| Lipids (%) | (m ± SD) | 38.0 ± 8.4 | 39.4 ± 5.3 | 35.9 ± 11.6 | NS |
| Lipids/kg of body weight | (m ± SD) | 2.1 ± 0.6 | 1.8 ± 0.4 | 2.4 ± 0.5 | <0.001 |
| Omega 3 fatty acids | (m ± SD) | 1.5 ± 1.1 | 1.08 ± 0.45 | 2.27 ± 1.38 | <0.01 |
| Omega 6/Omega 3 | (m ± SD) | 8.2 ± 4.2 | 10.1 ± 4.1 | 5.4 ± 2.3 | <0.001 |
| Vitamin A (UI) | (m ± SD) | 1364.5 ± 778.0 | 936.2 ± 526.7 | 2023.4 ± 631.7 | <0.001 |
| Vitamin D (UI) | (m ± SD) | 1187.1 ± 1670.5 | 532.5 ± 776.1 | 2194.1 ± 2162.2 | <0.05 |
| Vitamin E (mg) | (m ± SD) | 17.3 ± 8.1 | 13.4 ± 5.1 | 23.4 ± 8.2 | <0.01 |
| Vitamin K (mcg) | (m ± SD) | 138.8 ± 123.4 | 121.6 ± 130.5 | 182.5 ± 93.8 | NS |
| TOTAL FAT-SOLUBLE VITAMINS ** | |||||
| Vitamin A (UI) | (m ± SD) | 6403.9 ± 4379.8 | 5056.2 ± 4188.7 | 8477.2 ± 3961.1 | <0.05 |
| Vitamin D (UI) | (m ± SD) | 3471.0 ±2797.8 | 2370.7 ± 2169.4 | 5163.9 ± 2880.8 | <0.01 |
| Vitamin E (mg) | (m ± SD) | 267.3 ± 169.6 | 241.4 ± 179.1 | 307.2 ± 152.0 | |
| Vitamin K (mcg) | (m ± SD) | 1045.7 ± 1556.5 | 718.3 ± 716.7 | 1851.5 ± 2562.5 | <0.05 |
| CF | CF | ||||
|---|---|---|---|---|---|
| Total | No ONS (n = 46) | ONS (n = 13) | p | ||
| Blood test data (n = 59) | |||||
| Vitamin A (mcg/dl) | (m ± SD) | 46.4 ± 20.1 | 46.4 ± 19.8 | 46.6 ± 21.9 | NS |
| <20 | n (%) | 2 (3.4) | 1 (2.2) | 1 (7.7) | NS |
| 25-OH-Vitamin D (mcg/dl) | (m ± SD) | 38.1 ± 28.6 | 37.4 ± 31.3 | 40.6 ± 15.1 | NS |
| <20 | n (%) | 13 (22.0) | 13 (28.3) | 0 (0) | <0.05 |
| >30 | n (%) | 29 (49.2) | 21 (45.6) | 8 (61.5) | |
| Vitamin E (mg/g) | (m ± SD) | 1248.2 ± 413.8 | 1174.3 ± 396.6 | 1488.3 ± 390.9 | <0.05 |
| <800 | n (%) | 6 (10.2) | 6 (12.8) | 0 (0) | NS |
| Vitamin E/Cholesterol | (m ± SD) | 9.18 ± 3.30 | 8.3 ± 2.9 | 12.0 ± 3.0 | <0.001 |
| <5.7 | n (%) | 2 (3.4) | 2 (5.3) | 0 (0) | NS |
| Prothrombin time (%) | (m ± SD) | 97.1 ± 11.9 | 97.4 ± 11.8 | 95.7 ± 13.3 | NS |
| ucOC (ng/mL) | (m ± SD) | 4.7 ± 2.7 | 4.7 ± 2.7 | 4.7 ± 2.4 | NS |
| OC (ng/mL) | (m ± SD) | 26.4 ± 25.9 | 27.5 ± 26.7 | 22.0 ± 23.4 | NS |
| CTX (mcg/mL) | (m ± SD) | 0.55 ± 0.30 | 0.53 ± 0.30 | 0.63 ± 0.35 | NS |
| RANKL (pmol/L) | (m ± SD) | 0.23 ± 0.28 | 0.18 ± 0.22 | 0.42 ± 0.42 | <0.05 |
| CF | ||
|---|---|---|
| ONS (13) | ||
| DIETARY QUESTIONNAIRE | ||
| Mean ONS per day | (m ± SD) | 1.8 ± 1.1 |
| Mean calorie intake | (m ± SD) | 479.5 ± 326,1 |
| Calories from supplements (% of total) | (m ± SD) | 16.0 ± 10.4 |
| Carbohydrates (%) | (m ± SD) | 43.2 ± 6.6 |
| Carbohydrates (g) | (m ± SD) | 56.0 ± 39.9 |
| Proteins (%) | (m ± SD) | 16.9 ± 3.1 |
| Proteins (g) | (m ± SD) | 21.6 ± 13.9 |
| Lipids (%) | (m ± SD) | 40.0 ± 5.4 |
| Lipids (g) | (m ± SD) | 22.6 ± 14.4 |
| Omega 3 fatty acids | (m ± SD) | 1.1 ± 1.2 |
| Omega 6/Omega 3 | (m ± SD) | 0.3 ± 0.2 |
| Vitamin A (UI) | (m ± SD) | 560.2 ± 59.4 |
| Vitamin D (UI) | (m ± SD) | 219.6 ± 151.6 |
| Vitamin E (mg) | (m ± SD) | 10.5 ± 7.1 |
| Vitamin K (mcg) | (m ± SD) | 41.5 ± 31.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Bolívar, V.; Olveira, C.; Porras, N.; Abuín-Fernández, J.; García-Olivares, M.; Sánchez-Torralvo, F.J.; Girón, M.V.; Ruiz-García, I.; Olveira, G. Oral Nutritional Supplements in Adults with Cystic Fibrosis: Effects on Intake, Levels of Fat-Soluble Vitamins, and Bone Remodeling Biomarkers. Nutrients 2021, 13, 669. https://doi.org/10.3390/nu13020669
Contreras-Bolívar V, Olveira C, Porras N, Abuín-Fernández J, García-Olivares M, Sánchez-Torralvo FJ, Girón MV, Ruiz-García I, Olveira G. Oral Nutritional Supplements in Adults with Cystic Fibrosis: Effects on Intake, Levels of Fat-Soluble Vitamins, and Bone Remodeling Biomarkers. Nutrients. 2021; 13(2):669. https://doi.org/10.3390/nu13020669
Chicago/Turabian StyleContreras-Bolívar, Victoria, Casilda Olveira, Nuria Porras, José Abuín-Fernández, María García-Olivares, Francisco José Sánchez-Torralvo, María Victoria Girón, Ignacio Ruiz-García, and Gabriel Olveira. 2021. "Oral Nutritional Supplements in Adults with Cystic Fibrosis: Effects on Intake, Levels of Fat-Soluble Vitamins, and Bone Remodeling Biomarkers" Nutrients 13, no. 2: 669. https://doi.org/10.3390/nu13020669