The Recovery of a Sequencing Biofilm Batch Reactor—Anammox System: Performance, Metabolic Characteristics, and Microbial Community Analysis
Abstract
:1. Introduction
2. Materials and Methods
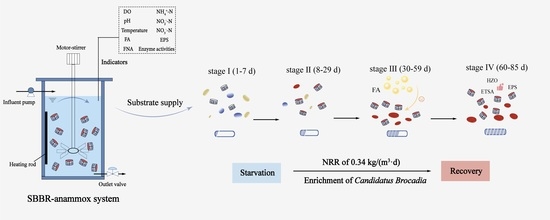
2.1. Experimental Set-Up
2.2. Substrate Composition and System Properties Analysis
2.3. Extracellular Polymeric Substances (EPS) Content
2.4. Metabolic Enzyme and Electron Transport System Activities
2.5. 16S rRNA Amplicon Sequencing
3. Results and Discussion
3.1. SBBR–Anammox System Performance
3.2. Variation in Metabolic Characteristics
3.2.1. Changes of EPS Contents
3.2.2. Metabolic Enzyme Activity
3.3. Microbial Community Analysis
3.3.1. Microbial Community Dynamics
3.3.2. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.Y.; Liu, L.J.; Bi, Y.M.; Meng, F.S.; Wang, D.; Qiu, C.S.; Yu, J.J.; Wang, S.P. A review of anammox metabolic response to environmental factors: Characteristics and mechanisms. Environ. Res. 2023, 223, 115464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Huo, T.R.; Sun, J.Q.; Feng, Y.M.; Pan, J.J.; Zhao, Y.P.; Liu, S.T. Response of amino acid metabolism to decreased temperatures in anammox consortia: Strong, efficient and flexible. Bioresour. Technol. 2022, 352, 127099. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Peng, Y.Z. Technical revolution of biological nitrogen removal from municipal wastewater: Recent advances in Anammox research and application. Sci. Sin. Technol. 2021, 52, 389–402. (In Chinese) [Google Scholar] [CrossRef]
- Zuo, L.S.; Yao, H.; Chen, H.; Li, H.Y.; Jia, F.X.; Guo, J.H. The application of glycine betaine to alleviate the inhibitory effect of salinity on one-stage partial nitritation/anammox process. Water Environ. Res. 2021, 93, 549–558. [Google Scholar] [CrossRef]
- Huang, D.-Q.; Wang, Y.; Wu, Q.; Chen, J.-R.; Li, Z.-Y.; Fan, N.-S.; Jin, N.-S. Anammox sludge preservation: Preservative agents, temperature and substrate. J. Environ. Manag. 2022, 311, 114860. [Google Scholar] [CrossRef]
- Huang, D.-Q.; Fu, J.-J.; Li, Z.-Y.; Fan, N.-S.; Jin, R.-C. Inhibition of wastewater pollutants on the anammox process: A review. Sci. Total Environ. 2022, 803, 150009. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Daverey, A.; Huang, Y.-T.; Sung, S.; Lin, J.-G. Treatment of semiconductor wastewater using single-stage partial nitrification and anammox in a pilot-scale reactor. J. Taiwan Inst. Chem. Eng. 2016, 63, 236–242. [Google Scholar] [CrossRef]
- Deng, W.F.; Wang, L.T.; Cheng, L.; Yang, W.B.; Gao, D.W. Nitrogen Removal from Mature Landfill Leachate via Anammox Based Processes: A Review. Sustainability 2022, 14, 995. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Chen, W.-Y. The Effect of Up-Flow Rate on the Nitrogen Treatment Efficiency and Sludge Characteristics of ANAMMOX Process with Up-Flow Anaerobic Sludge Bed Reactor. Sustainability 2022, 14, 16992. [Google Scholar] [CrossRef]
- Miao, L.; Wang, S.Y.; Cao, T.H.; Peng, Y.Z.; Zhang, M.; Liu, Z.Y. Advanced nitrogen removal from landfill leachate via Anammox system based on Sequencing Biofilm Batch Reactor (SBBR): Effective protection of biofilm. Bioresour. Technol. 2016, 220, 8–16. [Google Scholar] [CrossRef]
- Wu, P.; Chen, J.J.; Garlapati, V.K.; Zhang, X.X.; Jenario, F.W.V.; Li, X.; Liu, W.R.; Chen, C.J.; Aminabhavi, T.M.; Zhang, X.N. Novel insights into Anammox-based processes: A critical review. Chem. Eng. J. 2022, 444, 136534. [Google Scholar] [CrossRef]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Jin, R.C.; Yang, G.F.; Yu, J.J.; Zhen, P. The inhibition of the anammox process: A review. Chem. Eng. J. 2012, 197, 67–79. [Google Scholar] [CrossRef]
- Ji, Y.-X.; Jin, R.-C. Effect of different preservation conditions on the reactivation performance of anammox sludge. Sep. Purif. Technol. 2014, 133, 32–39. [Google Scholar] [CrossRef]
- Peng, Z.H.; Lei, Y.F.; Liu, Y.M.; Wan, X.; Yang, B.Q.; Pan, X.J. Fast start-up and reactivation of anammox process using polyurethane sponge. Biochem. Eng. J. 2022, 177, 108249. [Google Scholar] [CrossRef]
- Tian, X.; Schopf, A.; Amaral-Stewart, B.; Christensson, M.; Morgan-Sagastume, F.; Vincent, S.; Delatolla, R. Anammox attachment and biofilm development on surface-modified carriers with planktonic- and biofilm-based inoculation. Bioresour. Technol. 2020, 317, 124030. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Arroyo, J.M.; Puyol, D.; Li, G.B.; Swartwout, A.; Sierra-Álvarez, R.; Field, J.A. Starved anammox cells are less resistant to NO2− inhibition. Water Res. 2014, 65, 170–176. [Google Scholar] [CrossRef]
- Deng, Y.F.; Zhang, X.L.; Miao, Y.; Hu, B. Exploration of rapid start-up of the CANON process from activated sludge inoculum in a sequencing biofilm batch reactor (SBBR). Water Sci. Technol. 2016, 73, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.T.; Peng, Y.Z.; Zhang, J.H.; Zhang, L. Anammox bacteria enrichment in fixed biofilm successfully enhanced nitrogen removal of domestic wastewater in a sequencing biofilm batch reactor (SBBR). J. Water Pro. Eng. 2021, 42, 102154. [Google Scholar] [CrossRef]
- Laureni, M.; Weissbrodt, D.G.; Villez, K.; Robin, O.; Nadieh, D.J.; Rosenthal, A.; Wells, G.; Nielsen, J.L.; Morgenroth, E.; Joss, A. Biomass segregation between biofilm and flocs improves the control of nitrite-oxidizing bacteria in mainstream partial nitritation and anammox processes. Water Res. 2019, 154, 104–116. [Google Scholar] [CrossRef]
- Daverey, A.; Chen, Y.-C.; Dutta, K.; Huang, Y.-T.; Lin, J.-G. Start-up of Simultaneous Partial Nitrification, Anammox and Denitrification (SNAD) Process in Sequencing Batch Biofilm Reactor Using Novel Biomass Carriers. Bioresour. Technol. 2015, 190, 480–486. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, S.H.; Wu, P.; Lin, L.F.; Luo, H.Y. Start-up of the Canon process from activated sludge under salt stress in a sequencing batch biofilm reactor (SBBR). Bioresour. Technol. 2010, 101, 6309–6314. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-C.; Yu, T.; Gao, D.-W. Effects of HRT and nitrite/ammonia ratio on anammox discovered in a sequencing batch biofilm reactor. RSC Adv. 2014, 4, 54798–54804. [Google Scholar] [CrossRef]
- Hajsardar, M.; Borghei, S.M.; Hassani, A.H.; Takdastan, A. Nitrogen removal from ammonium-rich pharmaceutical wastewater. A comparison between sequencing batch reactor (SBR) and sequencing batch biofilm reactor (SBBR). Environ. Protect. Eng. 2018, 44, 95–115. [Google Scholar] [CrossRef]
- Mohan, S.V.; Rao, N.C.; Sarma, P.N. Low-biodegradable composite chemical wastewater treatment by biofilm configured sequencing batch reactor (SBBR). J. Hazard. Mater. 2007, 144, 108–117. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, L.; Miao, Y.Y.; Sun, Y.W.; Zhang, Q.; Wu, L.; Peng, Y.Z. Enhancing sewage nitrogen removal via anammox and endogenous denitrification: Significance of anaerobic/oxic/anoxic operation mode. Bioresour. Technol. 2019, 289, 121665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhang, L.; Miao, Y.Y.; Sun, Y.W.; Li, X.Y.; Zhang, Q.; Peng, Y.Z. Feasibility of in situ enriching anammox bacteria in a sequencing batch biofilm reactor (SBBR) for enhancing nitrogen removal of real domestic wastewater. Chem. Eng. J. 2018, 352, 847–854. [Google Scholar] [CrossRef]
- Niederdorfer, R.; Fragner, L.; Yuan, L.; Hausherr, D.; Wei, J.; Magyar, P.; Joss, A.; Lehmann, F.M.; Ju, F.; Bürgmann, H. Distinct growth stages controlled by the interplay of deterministic and stochastic processes in functional anammox biofilms. Water Res. 2021, 200, 117225. [Google Scholar] [CrossRef]
- Cai, F.R.; Lei, L.R.; Li, Y.M. Rapid start-up of single-stage nitrogen removal using anammox and partial nitritation (SNAP) process in a sequencing batch biofilm reactor (SBBR) inoculated with conventional activated sludge. Int. Biodeter. Biodegr. 2020, 147, 104877. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Q.; Wang, S.Y.; Li, B.K.; Wang, Z.; Zhang, S.J.; Zhang, M.; Peng, Y.Z. Characterization of EPS compositions and microbial community in an Anammox SBBR system treating landfill leachate. Bioresour. Technol. 2018, 249, 108–116. [Google Scholar] [CrossRef]
- Liu, W.; Nasry, A.A.N.B.; Zhao, J.Q.; Laoyongxay, H.; Dai, W.; Zhao, Q. Start-up of the Simultaneous Nitrification, Anammox, and Denitrification (SNAD) Reactor and Efficacy of a Small Amount of Organic Carbon. Water Air Soil Pollut. 2019, 230, 256. [Google Scholar] [CrossRef]
- Chen, K.Q.; Zhang, L.; Sun, S.H.; Li, J.W.; Jia, T.P.; Peng, Y.Z. In situ enrichment of anammox bacteria in anoxic biofilms are possible due to the stable and long-term accumulation of nitrite during denitrification. Bioresour. Technol. 2020, 300, 122668. [Google Scholar] [CrossRef]
- Rice, E.W.; Association, A. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Vázquez-Padín, J.R.; Pozo, M.J.; Jarpa, M.; Figueroa, M.; Franco, A.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Treatment of anaerobic sludge digester effluents by the CANON process in an air pulsing SBR. J. Hazard. Mater. 2009, 166, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Nishiyama, T.; Shigetomo, H.; Toyomoto, T.; Kawahara, Y.; Furukawa, K.; Fujii, T. Isolation of a multiheme protein with features of a hydrazine-oxidizing enzyme from an anaerobic ammonium-oxidizing enrichment culture. Appl. Environ. Microbiol. 2007, 73, 1065–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Fu, J.-J.; Wu, Q.-Y.; Chen, J.-Y.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. Build the expressway for the salt-tolerant anammox process: Acclimation strategy tells the story. J. Clean. Prod. 2021, 278, 123921. [Google Scholar] [CrossRef]
- Maurines-Carboneill, C.; Pernelle, J.-J.; Morin, L.; Sachon, G.; Leblon, G. Relevance of the INT test response as an indicator of ETS activity in monitoring heterotrophic aerobic bacterial populations in activated sludges. Water Res. 1998, 32, 1213–1221. [Google Scholar] [CrossRef]
- Kaewyai, J.; Noophan, P.; Wantawin, C.; Munakata-Marr, J. Recovery of enriched anammox biofilm cultures after storage at cold and room temperatures for 164 days. Int. Biodeter. Biodegr. 2019, 137, 1–7. [Google Scholar] [CrossRef]
- Ma, B.B.; Zhang, X.J.; Wei, D.H.; Ma, Y.P.; Wang, Q.; Zhang, H.; Zhang, J.J. Simultaneous nitrogen and sulfur removal using Anammox coupled sulfide-denitrification process: Impact of pH. J. Water Process Eng. 2022, 49, 103176. [Google Scholar] [CrossRef]
- Chang, G.W.; Yang, Y.; YYang, Y.F.; Yang, J.J.; Li, S.K.; Mu, X.Y.; Luo, J.W.; Li, X. Alleviation of substrate inhibition in an anaerobic ammonia oxidation reactor: Recovery process performance and microbial community. J. Water Process Eng. 2023, 51, 103439. [Google Scholar] [CrossRef]
- Yuan, H.P.; Zhu, N.W. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sust. Energ. Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Xiao, B.Y.; Liu, Y.; Luo, M.; Yang, T.; Guo, X.S.; Yi, H. Evaluation of the secondary structures of protein in the extracellular polymeric substances extracted from activated sludge by different methods. J. Environ. Sci. 2019, 80, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Liu, Y.; Niu, Q.G.; Ji, J.Y.; Hojo, T.; Li, Y.-Y. Nitrogen removal performance and loading capacity of a novel single-stage nitritation-anammox system with syntrophic micro-granules. Bioresour. Technol. 2017, 236, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.X.; Yang, Q.; Liu, X.H.; Li, X.Y.; Li, B.K.; Zhang, L.; Peng, Y.Z. Stratification of Extracellular Polymeric Substances (EPS) for Aggregated Anammox Microorganisms. Environ. Sci. Technol. 2017, 51, 3260–3268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Meng, F.G.; Zhou, C.Y.; Wu, X.W.; Chao, J. Optimum relative frequency and fluctuating substrate selection in reinforcing anammox-mediated anabolic adaptation. Water Res. 2022, 228, 119377. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, M.; Liu, K.; Yang, E.Z.; Chen, J.; Wu, S.; Xie, M.; Wang, D.B.; Deng, H.W.; Chen, H. Insights into the synergy between functional microbes and dissolved oxygen partition in the single-stage partial nitritation-anammox granules system. Bioresour. Technol. 2022, 347, 126364. [Google Scholar] [CrossRef]
- Ya, T.; Liu, J.Y.; Zhang, M.L.; Wang, Y.L.; Huang, Y.; Hai, R.T.; Zhang, T.T.; Wang, X.H. Metagenomic insights into the symbiotic relationship in anammox consortia at reduced temperature. Water Res. 2022, 225, 119184. [Google Scholar] [CrossRef]
- Wan, K.; Yu, Y.; Hu, J.G.; Liu, X.M.; Deng, X.Y.; Yu, J.X.; Chi, R.; Xiao, C.Q. Recovery of anammox process performance after substrate inhibition: Reactor performance, sludge morphology, and microbial community. Bioresour. Technol. 2022, 357, 127351. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; Li, D.; Li, S.; Zeng, H.P.; Zhang, J. Characteristics and mechanism of hollow anammox granular sludge with different settling properties. J. Environ. Chem. Eng. 2022, 10, 107230. [Google Scholar] [CrossRef]
- Corsino, S.F.; Capodici, M.; Torregrossa, M.; Viviani, G. Physical properties and Extracellular Polymeric Substances pattern of aerobic granular sludge treating hypersaline wastewater. Bioresour. Technol. 2017, 229, 152–159. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Bai, Y.-H.; Xia, W.-J.; Ni, S.-K.; Wu, Q.-Y.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. Exogenous extracellular polymeric substances as protective agents for the preservation of anammox granules. Sci. Total Environ. 2020, 747, 141764. [Google Scholar] [CrossRef]
- Lin, Q.J.; Kang, D.; Zhang, M.; Yu, T.; Xu, D.D.; Zeng, Z.; Zheng, P. The performance of anammox reactor during start-up: Enzymes tell the story. Process Saf. Environ. Protect. 2019, 121, 247–253. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Strous, M.; van de Pas-Schoonen, K.T.; Schalk, J.; van Dongen, U.G.J.M.; van de Graaf, A.A.; Logemann, S.; Muyzer, G.; van Loosdrecht, M.C.M.; Kuenen, J.G. The anaerobic oxidation of ammonium. Fems. Microbiol. Rev. 1998, 22, 421–437. [Google Scholar] [CrossRef]
- Podder, A.; Reinhart, D.; Goel, R. Nitrogen management in landfill leachate using single-stage anammox process-illustrating key nitrogen pathways under an ecogenomics framework. Bioresour. Technol. 2020, 312, 123578. [Google Scholar] [CrossRef]
- Vipindas, P.V.; Krishnan, K.P.; Rehitha, T.V.; Jabir, T.; Dinesh, S.L. Diversity of sediment associated Planctomycetes and its related phyla with special reference to anammox bacterial community in a high Arctic fjord. World J. Microb. Biot. 2020, 36, 107. [Google Scholar] [CrossRef]
- Lodha, T.; Narvekar, S.; Karodi, P. Classification of uncultivated anammox bacteria and Candidatus Uabimicrobium into new classes and provisional nomenclature as Candidatus Brocadiia classis nov. and Candidatus Uabimicrobiia classis nov. of the phylum Planctomycetes and novel family Candidatus Scalinduaceae fam. nov to accommodate the genus Candidatus Scalindua. Systemat. Appl. Microbiol. 2021, 44, 126272. [Google Scholar] [CrossRef]
- Chen, Z.J.; Meng, Y.B.; Sheng, B.B.; Zhou, Z.B.; Jin, C.; Meng, F.G. Linking Exoproteome Function and Structure to Anammox Biofilm Development. Environ. Sci. Technol. 2019, 53, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Wang, K.J.; Gong, H.; Yuan, Q.; Yang, M.J.; He, C.H.; Shi, C.; San, E.F. Integrating floc, aggregate and carrier to reap high-quality anammox biofilm. Bioresour. Technol. 2020, 309, 123325. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-M.; Zheng, C.; Wang, Y.-L.; Jin, R.-C. Decoding the interspecies interaction in anammox process with inorganic feeding through metagenomic and metatranscriptomic analysis. J. Clean. Prod. 2021, 288, 125961. [Google Scholar] [CrossRef]
- Qian, F.Y.; Gebreyesus, A.T.; Wang, J.F.; Shen, Y.L.; Liu, W.R.; Xie, L.L. Single-stage autotrophic nitrogen removal process at high loading rate: Granular reactor performance, kinetics, and microbial characterization. Appl. Microbiol. Biotechnol. 2018, 102, 2379–2389. [Google Scholar] [CrossRef]
- Zheng, B.Y.; Zhang, L.; Guo, J.H.; Zhang, S.J.; Yang, A.M.; Peng, Y.Z. Suspended sludge and biofilm shaped different anammox communities in two pilot-scale one-stage anammox reactors. Bioresour. Technol. 2016, 211, 273–279. [Google Scholar] [CrossRef]
- Oshiki, M.; Satoh, H.; Okabe, S. Ecology and physiology of anaerobic ammonium oxidizing bacteria. Environ. Microbiol. 2016, 18, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.; Lotti, T.; Kleerebezem, R.; Picioreanu, C.L.; van Loosdrecht, M.C.M. Outcompeting nitrite-oxidizing bacteria in single-stage nitrogen removal in sewage treatment plants: A model-based study. Water Res. 2014, 66, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, J.; Zhang, Y.L.; Liu, W.Z.; Wang, A.J. Exerting applied voltage promotes microbial activity of marine anammox bacteria for nitrogen removal in saline wastewater treatment. Water Res. 2022, 215, 118285. [Google Scholar] [CrossRef] [PubMed]






| Period | Water Inlet | Anaerobic Reaction | Sedimentation | Water Outlet | Idle |
|---|---|---|---|---|---|
| Time | 15 min | 360 min | 80 min | 15 min | 10 min |
| Samples | Reads | OTU (97%) | Shannon Index | Simpson Index |
|---|---|---|---|---|
| Stage I | 40,582.0 | 425.0 | 3.09 | 0.17 |
| Stage II | 48,778.0 | 487.0 | 3.45 | 0.11 |
| Stage III | 63,248.0 | 508.0 | 3.44 | 0.12 |
| Stage IV | 21,299.0 | 414.0 | 3.60 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, L.; Bi, Y.; Meng, F.; Wang, D.; Qiu, C.; Yu, J.; Wang, S. The Recovery of a Sequencing Biofilm Batch Reactor—Anammox System: Performance, Metabolic Characteristics, and Microbial Community Analysis. Sustainability 2023, 15, 10454. https://doi.org/10.3390/su151310454
Chen X, Liu L, Bi Y, Meng F, Wang D, Qiu C, Yu J, Wang S. The Recovery of a Sequencing Biofilm Batch Reactor—Anammox System: Performance, Metabolic Characteristics, and Microbial Community Analysis. Sustainability. 2023; 15(13):10454. https://doi.org/10.3390/su151310454
Chicago/Turabian StyleChen, Xiaoying, Lingjie Liu, Yanmeng Bi, Fansheng Meng, Dong Wang, Chunsheng Qiu, Jingjie Yu, and Shaopo Wang. 2023. "The Recovery of a Sequencing Biofilm Batch Reactor—Anammox System: Performance, Metabolic Characteristics, and Microbial Community Analysis" Sustainability 15, no. 13: 10454. https://doi.org/10.3390/su151310454