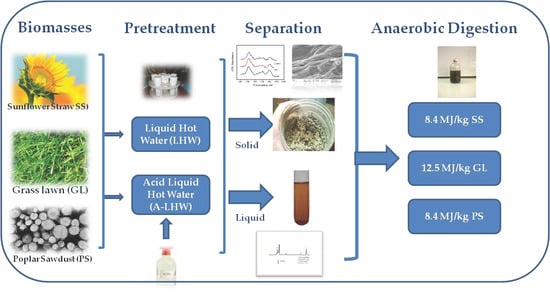
Does Acid Addition Improve Liquid Hot Water Pretreatment of Lignocellulosic Biomass towards Biohydrogen and Biogas Production?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Used
2.2. LHW and A-LHW Pretreatment
2.3. BMP and BHP Experimental Assays
2.4. Analytical Methods
2.5. Calculations
3. Results and Discussion
3.1. Impact of Pretreatments on Chemical Composition of Biomasses
3.2. Biofuel Generation
3.2.1. BHP of Pretreated Samples
3.2.2. BMP of Pretreated Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smullen, E.; Finnan, J.; Dowling, D.; Mulcahy, P. Bioconversion of switchgrass: Identification of a leading pretreatment option based on yield, cost and environmental impact. Renew. Energy 2017, 111, 638–645. [Google Scholar] [CrossRef]
- Antonopoulou, G. Designing efficient processes for sustainable bioethanol and bio-hydrogen production from grass lawn waste. Molecules 2020, 25, 2889. [Google Scholar] [CrossRef] [PubMed]
- Eskicioglu, C.; Monlau, F.; Barakat, A.; Ferrer, I.; Kaparaju, P.; Trably, E.; Carrère, H. Assessment of hydrothermal pretreatment of various lignocellulosic biomass with CO2 catalyst for enhanced methane and hydrogen production. Water Res. 2017, 120, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Ntaikou, I.; Lyberatos, G. Fungal pretreatment of willow sawdust with Abortiporus biennis for anaerobic digestion: Impact of an external nitrogen source. Sustainability 2017, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulou, G.; Vayenas, D.; Lyberatos, G. Ethanol and hydrogen production from sunflower straw: The effect of pretreatment on the whole slurry fermentation. Biochem. Eng. J. 2016, 116, 65–74. [Google Scholar] [CrossRef]
- Choiron, M.; Tojo, S.; Chosa, T. Biohydrogen production improvement using hot compressed water pretreatment on sake brewery waste. Int. J. Hydrog. Energy 2020, 45, 17220–17232. [Google Scholar] [CrossRef]
- Kucharska, K.; Hołowacz, I.; Konopacka-Łyskawa, D.; Rybarczyk, P.; Kamiński, M. Key issues in modeling and optimization of lignocellulosic biomass fermentative conversion to gaseous biofuels. Renew. Energy 2018, 129, 384–408. [Google Scholar] [CrossRef]
- Carrère, H.; Antonopoulou, G.; Passos, F.; Affes, R.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of pretreatment strategies for the most common anaerobic digestion feedstocks: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, O.A.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Wang, D.; Shena, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Luo, T.; Mei, Z. Can hydrothermal pretreatment improve anaerobic digestion for biogas from lignocellulosic biomass? Bioresour. Technol. 2018, 249, 117–124. [Google Scholar] [CrossRef]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Christakopoulos, P. Fermentation of liquefacted hydrothermally pretreated sweet sorghum bagasse to ethanol at high-solids content. Bioresour. Technol. 2013, 127, 202–208. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Dimitrellos, G.; Beobide, A.S.; Vayenas, D.; Lyberatos, G. Chemical pretreatment of sunflower straw biomass: The effect on chemical composition and structural changes. Waste Biomass Valor. 2015, 6, 733–746. [Google Scholar] [CrossRef]
- Owens, J.M.; Chynoweth, D.P. Biochemical methane potential of municipal solid waste (MSW) components. Water Sci. Technol. 1993, 27, 1–14. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Vayenas, D.; Lyberatos, G. Biogas production from physicochemically pretreated grass lawn waste: Comparison of different process schemes. Molecules 2020, 25, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater; Franson, M.A., Ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Alexandropoulou, M.; Antonopoulou, G.; Ntaikou, I.; Fragkou, E.; Lyberatos, G. Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J. Environ. Manag. 2017, 203, 704–713. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam aqueous pretreatments. Philos. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity—Relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- Ahmad, F.; Silva, E.L.; Varesche, M.B.A. Hydrothermal processing of biomass for anaerobic digestion—A review. Renew. Sustain. Energy Rev. 2018, 98, 108–124. [Google Scholar] [CrossRef]
- Monlau, F.; Kaparaju, P.; Trably, E.; Steyer, J.-P.; Carrère, H. Alkaline pretreatment to enhance one-stage CH4 and two-stage H2/CH4 production from sunflower stalks: Mass, energy and economical balances. Chem. Eng. J. 2015, 260, 377–385. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Karimi, K.; Mirmohamadsadeghi, S. Hydrothermal pretreatment of safflower straw to enhance biogas production. Energy 2019, 172, 545–554. [Google Scholar] [CrossRef]
- Xue, Y.; Li, G.; Gu, Y.; Yu, H.; Zhang, Y.; Zhou, X. Improving biodegradability and biogas production of miscanthus using a combination of hydrothermal and alkaline pretreatment. Ind. Crop. Prod. 2020, 144, 111985. [Google Scholar] [CrossRef]
- Shang, G.; Zhang, C.; Wang, F.; Qiu, L.; Guo, X.; Xu, F. Liquid hot water pretreatment to enhance the anaerobic digestion of wheat straw—Effects of temperature and retention time. Environ. Sci. Pollut. Res. 2019, 26, 29424–29434. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. The effect of aqueous ammonia soaking pretreatment on methane generation using different lignocellulosic biomasses. Waste Biomass Valor. 2015, 6, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Ghali, A.E.; Marzoug, I.B.; Baouab, M.H.V.; Roudesli, M.S. Separation and characterization of new cellulosic fibres from the Juncus acutus plant. BioResources 2012, 7, 2002–2018. [Google Scholar] [CrossRef]
- Yoo, C.G.; Kim, H.; Lu, F.; Azarpira, A.; Pan, X.; Oh, K.K.; Kim, J.S.; Ralph, J.; Kim, T.H. Understanding the physicochemical characteristics and the improved enzymatic saccharification of corn stover pretreated with aqueous and gaseous ammonia. BioEnergy Res. 2016, 9, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [Green Version]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Chandel, A.K.; Silva, S.S.; Singh, O.V. Detoxification of lignocellulosic hydrolysates for improved bioethanol production. In Biofuel Production Recent Developments and Prospects; Bernardes, M.A.D.S., Ed.; IntechOpen Limited: London, UK, 2011; pp. 225–246. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- González, L.; Reyes, I.; Dewulf, J.; Budde, J.; Heiermann, M.; Vervaeren, H. Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour. Technol. 2014, 169, 284–290. [Google Scholar] [CrossRef]
- Sun, T.S.; Wang, K.; Yang, G.; Yang, H.-Y.; Xu, F. Hydrothermal treatment and enzymatic saccharification of corn cobs. BioResources 2014, 9, 3000–3013. [Google Scholar] [CrossRef]
- Fang, C.J.; Schmidt, J.E.; Cybulska, I.; Brudecki, G.P.; Frankaer, C.G.; Thomsen, M.H. Hydrothermal pretreatment of date palm (Phoenix dactylifera L.) leaflets and rachis to enhance enzymatic digestibility and bioethanol potential. BioMed Res. Int. 2015, 216454. [Google Scholar] [CrossRef] [Green Version]
- Monlau, F.; Aemig, Q.; Trably, E.; Hamelin, J.; Steyer, J.-P.; Carrère, H. Specific inhibition of biohydrogen-producing Clostridium sp. after dilute-acid pretreatment of sunflower stalks. Int. J. Hydrog. Energy 2013, 38, 12273–12282. [Google Scholar] [CrossRef]
- Monlau, F.; Trably, E.; Barakat, E.; Hamelin, J.; Steyer, J.-P.; Carrère, H. Two-stage alkaline−enzymatic pretreatments to enhance biohydrogen production from sunflower stalks. Environ. Sci. Technol. 2013, 47, 12591–12599. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Alexandropoulou, M.; Ntaikou, I.; Lyberatos, G. From waste to fuel: Energy recovery from household food waste via its bioconversion to energy carriers based on microbiological processes. Sci. Total Environ. 2020, 732, 139230. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Angelidaki, I. Biogas production from ensiled meadow grass; effect of mechanical pretreatments and rapid determination of substrate biodegradability via physicochemical methods. Bioresour. Technol. 2015, 182, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Bule, M.; Ma, J.; Zhao, Q.; Frear, C.; Chen, S. Enhancing volatile fatty acid (VFA) and bio-methane production from lawn grass with pretreatment. Bioresour. Technol. 2014, 162, 243–249. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Stamatelatou, K.; Lyberatos, G. Exploitation of rapeseed residues and sunflower residues for the methane generation through anaerobic digestion: The effect of pretreatment. Chem. Eng. Trans. 2010, 20, 253–258. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Steyer, J.-P.; Carrère, H. Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour. Technol. 2012, 120, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cegri, V.; De la Rubia, M.A.; Raposo, F.; Borja, R. Effect of hydrothermal pretreatment of sunflower oil cake on biomethane potential focusing on fibre composition. Bioresour. Technol. 2012, 123, 424–429. [Google Scholar] [CrossRef]





| Total Soluble Sugars | Glucose | Xylose | Arabinose | ||
|---|---|---|---|---|---|
| SS | LHW | 3.11 ± 0.19 | 0.52 ± 0.02 | 0.46 ± 0.00 | 0.87 ± 0.01 |
| A-LHW | 3.45 ± 0.38 | 0.56 ± 0.05 | 0.87 ± 0.03 | 1.22 ± 0.01 | |
| GL | LHW | 7.34 ± 0.14 | 1.18 ± 0.08 | 2.63 ± 0.06 | 2.46 ± 0.05 |
| A-LHW | 8.83 ± 0.63 | 1.25 ± 0.05 | 2.87 ± 0.03 | 2.99 ± 0.01 | |
| PS | LHW | 2.00 ± 0.16 | 0.15 ± 0.02 | 0.16 ± 0.06 | 0.26 ± 0.05 |
| A-LHW | 2.07 ± 0.29 | 0.15 ± 0.03 | 0.16 ± 0.02 | 0.28 ± 0.05 |
| Formic Acid | Acetic Acid | 5-HMF | Furfural | ||
|---|---|---|---|---|---|
| SS | LHW | 1.98 ± 0.08 | 1.52 ± 0.07 | 0.05 ± 0.00 | 0.15 ± 0.03 |
| A-LHW | 2.20 ± 0.06 | 1.56 ± 0.05 | 0.07 ± 0.01 | 0.27 ± 0.04 | |
| GL | LHW | 0.00 ± 0.00 | 1.09 ± 0.06 | 0.02 ± 0.00 | 0.51 ± 0.04 |
| A-LHW | 0.00 ± 0.00 | 1.02 ± 0.04 | 0.15 ± 0.01 | 0.92 ± 0.08 | |
| PS | LHW | 0.23 ± 0.01 | 0.62 ± 0.01 | 0.05 ± 0.01 | 0.22 ± 0.01 |
| A-LHW | 0.25 ± 0.02 | 0.65 ± 0.01 | 0.12 ± 0.03 | 0.75 ± 0.04 |
| H2 (mL/g VSinitial) | Energy (MJ/kg TSinitial) | ||
|---|---|---|---|
| Raw | 74.9 ± 5.2 | 0.8 | |
| SS | LHW | 154.1 ± 15.1 | 1.6 |
| A-LHW | 151.4 ± 15.0 | 1.5 | |
| Raw | 86.3 ± 7.8 | 0.9 | |
| GL | LHW | 103.4 ± 4.6 | 1.1 |
| A-LHW | 114.4 ± 9.6 | 1.2 | |
| Raw | 89.3 ± 4.3 | 1.0 | |
| PS | LHW | 94.6 ± 15.4 | 1.1 |
| A-LHW | 70.5 ± 5.9 | 0.8 |
| Solid Fraction | Liquid Fraction | Total | ||||
|---|---|---|---|---|---|---|
| BMP (mL/g VSinitial) | Energy (MJ/kg TSinitial) | BMP (mL/g VSinitial) | Energy (MJ/kg TSinitial) | Energy (MJ/kg TSinitial) H2-CH4/CH4-CH4 Concept | ||
| Raw a | 272.9 ± 16.3 | 8.6 | 0.8 b/8.6 c | |||
| SS | LHW | 161.2 ± 9.4 | 5.1 | 105.1 ± 0.4 | 3.3 | 4.9/8.4 |
| A-LHW | 121.4 ± 4.9 | 3.8 | 75.1 ± 0.3 | 2.4 | 3.9/6.2 | |
| Raw a | 339.9 ± 1.2 | 11.2 | 0.9 b/11.2 c | |||
| GL | LHW | 225.9 ± 0.8 | 7.4 | 153.6 ± 17.6 | 5.1 | 6.2/12.5 |
| A-LHW | 181.2 ± 17.0 | 5.6 | 101.0 ± 3.9 | 3.3 | 4.5/8.9 | |
| Raw a | 140.2 ± 13.08 | 5.1 | 1.0 b/5.1 c | |||
| PS | LHW | 177.2 ± 17.8 | 6.5 | 26.8 ± 3.7 | 1.0 | 2.1/7.5 |
| A-LHW | 123.4 ± 12.7 | 4.5 | 10.1 ± 1.0 | 0.4 | 1.2/4.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrellos, G.; Lyberatos, G.; Antonopoulou, G. Does Acid Addition Improve Liquid Hot Water Pretreatment of Lignocellulosic Biomass towards Biohydrogen and Biogas Production? Sustainability 2020, 12, 8935. https://doi.org/10.3390/su12218935
Dimitrellos G, Lyberatos G, Antonopoulou G. Does Acid Addition Improve Liquid Hot Water Pretreatment of Lignocellulosic Biomass towards Biohydrogen and Biogas Production? Sustainability. 2020; 12(21):8935. https://doi.org/10.3390/su12218935
Chicago/Turabian StyleDimitrellos, George, Gerasimos Lyberatos, and Georgia Antonopoulou. 2020. "Does Acid Addition Improve Liquid Hot Water Pretreatment of Lignocellulosic Biomass towards Biohydrogen and Biogas Production?" Sustainability 12, no. 21: 8935. https://doi.org/10.3390/su12218935