Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury
Abstract
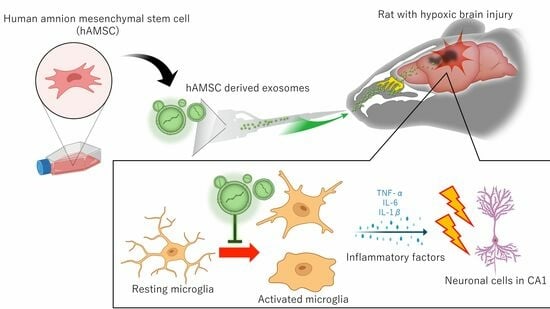
:1. Introduction
2. Methods
2.1. Stem Cell Preparation and Exosome Extraction
2.2. Animals Models of HIBI and Exosome Administration
2.3. Intranasal Administration of Exosomes
2.4. Neurological Assessments
2.5. Exosome Absorption and Distribution
2.6. Immunohistochemistry
2.7. In Vitro Microglial Assay
2.8. Statistical Analyses
3. Results
3.1. Animals Welfare
3.2. Exosome Characterization
3.3. Intranasal Exosome Administration Ameliorates Short- and Long-Term Memory Impairment
3.4. Biodistribution of Exosome
3.5. Exosome Ameliorate Neuronal Damage in CA1
3.6. Microglial Inflammation Were Inhibited by Intranasal Exosome Administration
3.7. Inhibitory Role of Exosome for Microglial Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosseini, M.; Wilson, R.H.; Crouzet, C.; Amirhekmat, A.; Wei, K.S.; Akbari, Y. Resuscitating the Globally Ischemic Brain: Ttm and Beyond. Neurotherapeutics 2020, 17, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Geocadin, R.G.; Wijdicks, E.; Armstrong, M.J.; Damian, M.; Mayer, S.A.; Ornato, J.P.; Rabinstein, A.; Suarez, J.I.; Torbey, M.T.; Dubinsky, R.M.; et al. Practice Guideline Summary: Reducing Brain Injury Following Cardiopulmonary Resuscitation: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2017, 88, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Callaway, C.W.; Haywood, K.; Neumar, R.W.; Lilja, G.; Rowland, M.J.; Sawyer, K.N.; Skrifvars, M.B.; Nolan, J.P. Brain Injury after Cardiac Arrest. Lancet 2021, 398, 1269–1278. [Google Scholar] [CrossRef]
- Liu, X.; Jia, X. Neuroprotection of Stem Cells against Ischemic Brain Injury: From Bench to Clinic. Transl. Stroke Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating Microrna in Body Fluid: A New Potential Biomarker for Cancer Diagnosis and Prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-Mediated Transfer of Mrnas and Micrornas Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Choi, H.; Kim, M.Y.; Kim, D.H.; Yun, H.; Oh, B.K.; Kim, S.B.; Song, I.H.; Park, H.S.; Kim, S.E.; Park, C.; et al. Quantitative Biodistribution and Pharmacokinetics Study of Gmp-Grade Exosomes Labeled with (89)Zr Radioisotope in Mice and Rats. Pharmaceutics 2022, 14, 1118. [Google Scholar] [CrossRef]
- Kawabori, M.; Kuroda, S.; Sugiyama, T.; Ito, M.; Shichinohe, H.; Houkin, K.; Kuge, Y.; Tamaki, N. Intracerebral, but Not Intravenous, Transplantation of Bone Marrow Stromal Cells Enhances Functional Recovery in Rat Cerebral Infarct: An Optical Imaging Study. Neuropathology 2012, 32, 217–226. [Google Scholar] [CrossRef]
- Gotoh, S.; Kawabori, M.; Fujimura, M. Intranasal Administration of Stem Cell-Derived Exosomes for Central Nervous System Diseases. Neural Regen. Res. 2024, 19, 1249–1255. [Google Scholar] [CrossRef]
- Takamiya, S.; Kawabori, M.; Yamazaki, K.; Yamaguchi, S.; Tanimori, A.; Yamamoto, K.; Ohnishi, S.; Seki, T.; Konno, K.; Tha, K.K.; et al. Intravenous Transplantation of Amnion-Derived Mesenchymal Stem Cells Promotes Functional Recovery and Alleviates Intestinal Dysfunction after Spinal Cord Injury. PLoS ONE 2022, 17, e0270606. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mikami, D.; Mioka, T.; Mukai, K.; Igarashi, Y. Lysosomal-Associated Transmembrane Protein 4b Regulates Ceramide-Induced Exosome Release. FASEB J. 2020, 34, 16022–16033. [Google Scholar] [CrossRef]
- Yuyama, K.; Takahashi, K.; Usuki, S.; Mikami, D.; Sun, H.; Hanamatsu, H.; Furukawa, J.; Mukai, K.; Igarashi, Y. Plant Sphingolipids Promote Extracellular Vesicle Release and Alleviate Amyloid-Beta Pathologies in a Mouse Model of Alzheimer’s Disease. Sci. Rep. 2019, 9, 16827. [Google Scholar] [CrossRef]
- Lu, D.; Wu, Y.; Qu, Y.; Shi, F.; Hu, J.; Gao, B.; Wang, B.; Gao, G.; He, S.; Zhao, T. A Modified Method to Reduce Variable Outcomes in a Rat Model of Four-Vessel Arterial Occlusion. Neurol. Res. 2016, 38, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Thomi, G.; Joerger-Messerli, M.; Haesler, V.; Muri, L.; Surbek, D.; Schoeberlein, A. Intranasally Administered Exosomes from Umbilical Cord Stem Cells Have Preventive Neuroprotective Effects and Contribute to Functional Recovery after Perinatal Brain Injury. Cells 2019, 8, 855. [Google Scholar] [CrossRef] [PubMed]
- Kurzina, N.P.; Aristova, I.Y.; Volnova, A.B.; Gainetdinov, R.R. Deficit in Working Memory and Abnormal Behavioral Tactics in Dopamine Transporter Knockout Rats during Training in the 8-Arm Maze. Behav. Brain Res. 2020, 390, 112642. [Google Scholar] [CrossRef]
- Kawabori, M.; Hokari, M.; Zheng, Z.; Kim, J.Y.; Calosing, C.; Hsieh, C.L.; Nakamura, M.C.; Yenari, M.A. Triggering Receptor Expressed on Myeloid Cells-2 Correlates to Hypothermic Neuroprotection in Ischemic Stroke. Ther. Hypothermia Temp. Manag. 2013, 3, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ikenari, T.; Kurata, H.; Satoh, T.; Hata, Y.; Mori, T. Evaluation of Fluoro-Jade C Staining: Specificity and Application to Damaged Immature Neuronal Cells in the Normal and Injured Mouse Brain. Neuroscience 2020, 425, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kawabori, M.; Houkin, K. Fty720 (Fingolimod) Ameliorates Brain Injury through Multiple Mechanisms and Is a Strong Candidate for Stroke Treatment. Curr. Med. Chem. 2019, 27, 2979–2993. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kawabori, M.; Seki, T.; Takamiya, S.; Tateno, T.; Konno, K.; Watanabe, M.; Houkin, K. Fty720 Attenuates Neuropathic Pain after Spinal Cord Injury by Decreasing Systemic and Local Inflammation in a Rat Spinal Cord Compression Model. J. Neurotrauma 2020, 37, 1720–1728. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Guo, R.; Guo, J.; Tian, X.; Wang, J.; Sun, S.; Han, Y.; Wang, Y. Exosomal Mir-23b-3p from Bone Mesenchymal Stem Cells Alleviates Experimental Autoimmune Encephalomyelitis by Inhibiting Microglial Pyroptosis. Exp. Neurol. 2023, 363, 114374. [Google Scholar] [CrossRef]
- Zook, N.; Voss, S.; Nordstrom, E.B.; Brett, S.J.; Jenkinson, E.; Shaw, P.; White, P.; Benger, J. Neurocognitive Function Following out-of-Hospital Cardiac Arrest: A Systematic Review. Resuscitation 2022, 170, 238–246. [Google Scholar] [CrossRef]
- Moulaert, V.R.; Verbunt, J.A.; van Heugten, C.M.; Wade, D.T. Cognitive Impairments in Survivors of out-of-Hospital Cardiac Arrest: A Systematic Review. Resuscitation 2009, 80, 297–305. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Gu, H.; Wang, D.; Guan, T.; Liao, W.; Peng, X. Prognostic Value of Magnetic Resonance Imaging Performed during the Subacute Phase in Adult Patients with Hypoxic-Ischemic Encephalopathy for Long-Term Neurological Outcomes. J. Stroke Cerebrovasc. Dis. 2020, 29, 104950. [Google Scholar] [CrossRef] [PubMed]
- Byron-Alhassan, A.; Tulloch, H.E.; Collins, B.; Quinlan, B.; Fang, Z.; Chakraborty, S.; Le May, M.; Duchesne, L.; Smith, A.M. Exploratory Analyses of Cerebral Gray Matter Volumes after out-of-Hospital Cardiac Arrest in Good Outcome Survivors. Front. Psychol. 2020, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The Role of Serotonin in Fear Learning and Memory: A Systematic Review of Human Studies. Brain Sci. 2023, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Spekker, E.; Szabo, A.; Polyak, H.; Vecsei, L. Modelling the Neurodevelopmental Pathogenesis in Neuropsychiatric Disorders. Bioactive Kynurenines and Their Analogues as Neuroprotective Agents-in Celebration of 80th Birthday of Professor Peter Riederer. J. Neural Transm. 2022, 129, 627–642. [Google Scholar] [CrossRef]
- Saccaro, L.F.; Schilliger, Z.; Perroud, N.; Piguet, C. Inflammation, Anxiety, and Stress in Attention-Deficit/Hyperactivity Disorder. Biomedicines 2021, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T.; Dohring, J.; Reuter, S.; Finke, C.; Rohr, A.; Brauer, H.; Deuschl, G.; Jansen, O. Selective Neuronal Vulnerability of Human Hippocampal Ca1 Neurons: Lesion Evolution, Temporal Course, and Pattern of Hippocampal Damage in Diffusion-Weighted Mr Imaging. J. Cereb. Blood Flow Metab. 2015, 35, 1836–1845. [Google Scholar] [CrossRef]
- Meyer, P.; Grandgirard, D.; Lehner, M.; Haenggi, M.; Leib, S.L. Grafted Neural Progenitor Cells Persist in the Injured Site and Differentiate Neuronally in a Rodent Model of Cardiac Arrest-Induced Global Brain Ischemia. Stem. Cells Dev. 2020, 29, 574–585. [Google Scholar] [CrossRef]
- Wi, S.; Yu, J.H.; Kim, M.; Cho, S.R. In Vivo Expression of Reprogramming Factors Increases Hippocampal Neurogenesis and Synaptic Plasticity in Chronic Hypoxic-Ischemic Brain Injury. Neural Plast. 2016, 2016, 2580837. [Google Scholar] [CrossRef]
- Nakano, M.; Nagaishi, K.; Konari, N.; Saito, Y.; Chikenji, T.; Mizue, Y.; Fujimiya, M. Bone Marrow-Derived Mesenchymal Stem Cells Improve Diabetes-Induced Cognitive Impairment by Exosome Transfer into Damaged Neurons and Astrocytes. Sci. Rep. 2016, 6, 24805. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Qian, Z.; Wang, M.; Zhang, F.; Zeng, T.; Li, L.; Gao, L. Exosomes from Adscs Ameliorate Nerve Damage in the Hippocampus Caused by Post Traumatic Brain Injury Via the Delivery of Circ-Scmh1 Promoting Microglial M2 Polarization. Injury 2023, 54, 110927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gan, Y.; Xu, G.; Hua, K.; Liu, D. Exosomes from Mscs Overexpressing Microrna-223-3p Attenuate Cerebral Ischemia through Inhibiting Microglial M1 Polarization Mediated Inflammation. Life Sci. 2020, 260, 118403. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tu, Z.; Yang, D.; Hu, M.; Zhou, L.; Li, Q.; Yu, B.; Hou, S. Exosomes from Hypoxic Pre-Treated Adscs Attenuate Acute Ischemic Stroke-Induced Brain Injury Via Delivery of Circ-Rps5 and Promote M2 Microglia/Macrophage Polarization. Neurosci. Lett. 2022, 769, 136389. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Lin, M.C.; Tsai, J.S.; He, P.L.; Luo, W.T.; Chiu, I.M.; Herschman, H.R.; Li, H.J. Exosomal 2′,3′-Cnp from Mesenchymal Stem Cells Promotes Hippocampus Ca1 Neurogenesis/Neuritogenesis and Contributes to Rescue of Cognition/Learning Deficiencies of Damaged Brain. Stem Cells Transl. Med. 2020, 9, 499–517. [Google Scholar] [CrossRef]
- Herman, S.; Fishel, I.; Offen, D. Intranasal Delivery of Mesenchymal Stem Cells-Derived Extracellular Vesicles for the Treatment of Neurological Diseases. Stem Cells 2021, 39, 1589–1600. [Google Scholar] [CrossRef]
- Tolomeo, A.M.; Zuccolotto, G.; Malvicini, R.; De Lazzari, G.; Penna, A.; Franco, C.; Caicci, F.; Magarotto, F.; Quarta, S.; Pozzobon, M.; et al. Biodistribution of Intratracheal, Intranasal, and Intravenous Injections of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles in a Mouse Model for Drug Delivery Studies. Pharmaceutics 2023, 15, 548. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal Delivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Ma, X.; Huang, M.; Zheng, M.; Dai, C.; Song, Q.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. Adscs-Derived Extracellular Vesicles Alleviate Neuronal Damage, Promote Neurogenesis and Rescue Memory Loss in Mice with Alzheimer’s Disease. J. Control Release 2020, 327, 688–702. [Google Scholar] [CrossRef]
- Rohden, F.; Teixeira, L.V.; Bernardi, L.P.; Ferreira, P.C.L.; Colombo, M.; Teixeira, G.R.; de Oliveira, F.D.; Lima, E.O.C.; Guma, F.C.R.; Souza, D.O. Functional Recovery Caused by Human Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles Administered 24 H after Stroke in Rats. Int. J. Mol. Sci. 2021, 22, 12860. [Google Scholar] [CrossRef]
- Dehghani, M.; Gulvin, S.M.; Flax, J.; Gaborski, T.R. Systematic Evaluation of Pkh Labelling on Extracellular Vesicle Size by Nanoparticle Tracking Analysis. Sci. Rep. 2020, 10, 9533. [Google Scholar] [CrossRef] [PubMed]
- Puzar Dominkus, P.; Stenovec, M.; Sitar, S.; Lasic, E.; Zorec, R.; Plemenitas, A.; Zagar, E.; Kreft, M.; Lenassi, M. Pkh26 Labeling of Extracellular Vesicles: Characterization and Cellular Internalization of Contaminating Pkh26 Nanoparticles. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Holthaus, D. Illinois Denies Tax Exemptions to Subsidiaries. Hospitals 1988, 62, 57. [Google Scholar]
- Wang, Y.; Niu, H.; Li, L.; Han, J.; Liu, Z.; Chu, M.; Sha, X.; Zhao, J. Anti-Chac1 Exosomes for Nose-to-Brain Delivery of Mir-760-3p in Cerebral Ischemia/Reperfusion Injury Mice Inhibiting Neuron Ferroptosis. J. Nanobiotechnol. 2023, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Madhu, L.N.; Attaluri, S.; Gitai, D.L.G.; Pinson, M.R.; Kodali, M.; Shetty, G.; Zanirati, G.; Kumar, S.; Shuai, B.; et al. Extracellular Vesicles from Human Ipsc-Derived Neural Stem Cells: Mirna and Protein Signatures, and Anti-Inflammatory and Neurogenic Properties. J. Extracell. Vesicles 2020, 9, 1809064. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabo, A.; Vecsei, L.; Gimenez-Llort, L. Emerging Translational Research in Neurological and Psychiatric Diseases: From In Vitro to In Vivo Models. Int. J. Mol. Sci. 2023, 24, 15739. [Google Scholar] [CrossRef] [PubMed]
- Morishima, Y.; Kawabori, M.; Yamazaki, K.; Takamiya, S.; Yamaguchi, S.; Nakahara, Y.; Senjo, H.; Hashimoto, D.; Masuda, S.; Fujioka, Y.; et al. Intravenous Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Spinal Cord Injury by Regulating Neutrophil Extracellular Trap Formation through Exosomal Mir-125a-3p. Int. J. Mol. Sci. 2024, 25, 2406. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, T.; Kawabori, M.; Zheng, Y.; Yamaguchi, S.; Gotoh, S.; Nakahara, Y.; Yoshie, E.; Fujimura, M. Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury. Pharmaceutics 2024, 16, 446. https://doi.org/10.3390/pharmaceutics16040446
Ikeda T, Kawabori M, Zheng Y, Yamaguchi S, Gotoh S, Nakahara Y, Yoshie E, Fujimura M. Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury. Pharmaceutics. 2024; 16(4):446. https://doi.org/10.3390/pharmaceutics16040446
Chicago/Turabian StyleIkeda, Takuma, Masahito Kawabori, Yuyuan Zheng, Sho Yamaguchi, Shuho Gotoh, Yo Nakahara, Erika Yoshie, and Miki Fujimura. 2024. "Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury" Pharmaceutics 16, no. 4: 446. https://doi.org/10.3390/pharmaceutics16040446