Advantages and Prospective Implications of Smart Materials in Tissue Engineering: Piezoelectric, Shape Memory, and Hydrogels
Abstract
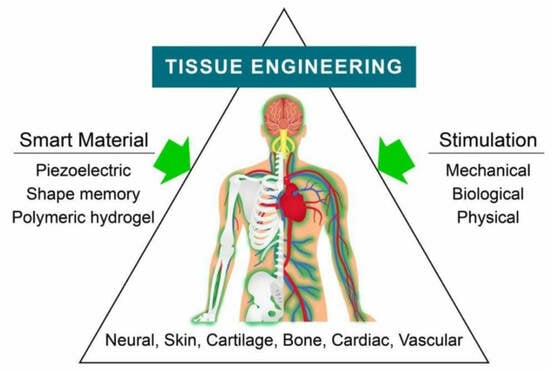
:1. Introduction
2. Protein-Based Smart Materials in Tissue Engineering
2.1. Piezoelectric Material
2.2. Shape-Memory Material
2.3. Polymeric Hydrogels
3. Advantages of Protein-Based Smart Materials in Tissue Engineering
4. Future Prospects of Smart Materials in Tissue Engineering
5. Conclusions and Way Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jingcheng, L.; Reddy, V.S.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Ramakrishna, S.; Ghosh, R. Intelligent polymers, fibers and applications. Polymers 2021, 13, 1427. [Google Scholar] [CrossRef]
- Mrinalini, M.; Prasanthkumar, S. Recent advances on stimuli-responsive smart materials and their applications. ChemPlusChem 2019, 84, 1103–1121. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.G.; Marino, A.; Tapeinos, C.; Ciofani, G. Smart materials meet multifunctional biomedical devices: Current and prospective implications for nanomedicine. Front. Bioeng. Biotechnol. 2017, 5, 80. [Google Scholar] [CrossRef]
- Kamila, S. Introduction, classification and applications of smart materials: An overview. Am. J. Appl. Sci. 2013, 10, 876–880. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Shehata, N.; Abdelkareem, M.A.; Sayed, E.T.; Egirani, D.E.; Opukumo, A.W. Smart Materials: The Next Generation. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Wang, L.; Huang, X. New protein-based smart materials. In Artificial Protein and Peptide Nanofibers; Woodhead Publishing: Cambridge, UK, 2020; pp. 415–436. [Google Scholar]
- Abascal, N.C.; Regan, L. The past, present and future of protein-based materials. R. Soc. Open Biol. 2018, 8, 180113. [Google Scholar] [CrossRef]
- Qian, Z.G.; Pan, F.; Xia, X.X. Synthetic biology for protein-based materials. Curr. Opin. Biotechnol. 2020, 65, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-responsive drug release from smart polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Phillips, G.J. Biomimetic, Bioresponsive, and Bioactive Materials: An Introduction to Integrating Materials with Tissues; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef]
- Morris, E.; Chavez, M.; Tan, C. Dynamic biomaterials: Toward engineering autonomous feedback. Curr. Opin. Biotechnol. Syst. Biol.-Nanobiotechnol. 2016, 39, 97–104. [Google Scholar] [CrossRef]
- Brighenti, R.; Li, Y.; Vernerey, F.J. Smart polymers for advanced applications: A mechanical perspective review. Front. Mater. 2020, 7, 196. [Google Scholar] [CrossRef]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef]
- Greco, F.; Mattoli, V. Introduction to Active Smart Materials for Biomedical Applications. In Piezoelectric Nanomaterials for Biomedical Applications, Nanomedicine and Nanotoxicology; Ciofani, G., Menciassi, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–27. [Google Scholar]
- Thangudu, S. Next Generation Nanomaterials: Smart Nanomaterials, Significance, and Biomedical Applications. In Applications of Nanomaterials in Human Health; Khan, F.A., Ed.; Springer: Singapore, 2020; pp. 287–312. [Google Scholar]
- Kapusetti, G.; More, N.; Choppadandi, M. Introduction to Ideal Characteristics and Advanced Biomedical Applications of Biomaterials. In Biomedical Engineering and Its Applications in Healthcare; Paul, S., Ed.; Springer: Singapore, 2019; pp. 171–204. [Google Scholar]
- Tandon, B.; Blaker, J.J.; Cartmell, S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018, 73, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Y.; Sun, H. Protein-based biomaterials for bone tissue regeneration. Front. Bioeng. Biotechnol. 2019, 7, 294. [Google Scholar]
- Jarkov, V.; Allan, S.J.; Bowen, C.; Khanbareh, H. Piezoelectric materials and systems for tissue engineering and implantable energy harvesting devices for biomedical applications. Int. Mater. Rev. 2022, 67, 683–733. [Google Scholar] [CrossRef]
- Zaszczynska, A.; Sajkiewicz, P.; Gradys, A. Piezoelectric scaffolds as smart materials for neural tissue engineering. Polymers 2020, 12, 161. [Google Scholar] [CrossRef]
- Miyamoto, S.; Shoji, T.; Miyachi, H.; Shinoka, T. Smart Biomaterials for Cardiovascular Tissue Engineering. In RSC Smart Materials; RSC Publishing: Cambridge, UK, 2017; pp. 230–257. [Google Scholar]
- Rosso, F.; Marino, G.; Giordano, A.; Barbarisi, M.; Parmeggiani, D.; Barbarisi, A. Smart materials as scaffolds for tissue engineering. J. Cell. Physiol. 2005, 203, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Kaliva, M.; Chatzinikolaidou, M.; Vamvakaki, M. Chapter 1 Applications of smart multifunctional tissue engineering scaffolds. In Smart Materials for Tissue Engineering; Royal Society of Chemistry: London, UK, 2017; pp. 1–38. [Google Scholar]
- Xie, M.; Wang, L.; Ge, J.; Guo, B.; Ma, P.X. Strong electroactive biodegradable shape memory polymer networks based on star-shaped polylactide and aniline trimer for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 6772–6781. [Google Scholar] [CrossRef]
- Kumar, C.; Aradhya, K.S.S. Smart Material Verses Conventional Materials in Biomedical Application Especially in Articular Surface Replacement. In Proceedings of the National Conference on Computational Methods in Mechanical Engineering, Trondheim, Norway, 11–12 May 2005. [Google Scholar]
- Wadood, A. Brief overview on Nitinol as biomaterial. Adv. Mater. Sci. Eng. 2016, 2016, e4173138. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Rodrigues, I.C.P.; Perez, A.G.M.; Souto, E.M.B.; Gabriel, L.P.; Webster, T. Scaffolds for Tissue Engineering: A State-of-the-Art Review Concerning Types, Properties, Materials, Processing, and Characterization. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 647–676. [Google Scholar]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Wang, H.; Jasim, A. Piezoelectric Energy Harvesting from Pavement. In Eco-Efficient Pavement Construction Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 367–382. [Google Scholar]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured biomaterials for bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric biomaterials for sensors and actuators. Adv. Mater. 2019, 31, 1802084. [Google Scholar] [CrossRef]
- Lu, Y.J.; Shi, Z.F.; Shan, C.X.; Shen, D.Z. Chapter 4-ZnO Nanostructures and Lasers. In Nanoscale Semiconductor Lasers, Micro and Nano Technologies; Tong, C., Jagadish, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 75–108. [Google Scholar]
- Tofail, S.A.M. Pyroelectricity in biological materials and biomaterials: A five decades long journey. Ferroelectrics 2014, 472, 11–18. [Google Scholar] [CrossRef]
- Lang, S.B.; Tofail, S.A.M.; Kholkin, A.L.; Wojtaś, M.; Gregor, M.; Gandhi, A.A.; Wang, Y.; Bauer, S.; Krause, M.; Plecenik, A. Ferroelectric polarization in nanocrystalline hydroxyapatite thin films on silicon. Sci. Rep. 2013, 3, 2215. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.-H.; Kim, J. A review of piezoelectric energy harvesting based on vibration. Int. J. Precis. Eng. Manuf. 2011, 12, 1129–1141. [Google Scholar] [CrossRef]
- Mishra, S.; Unnikrishnan, L.; Nayak, S.K.; Mohanty, S. Advances in piezoelectric polymer composites for energy harvesting applications: A systematic review. Macromol. Mater. Eng. 2019, 304, 1800463. [Google Scholar] [CrossRef]
- Zheng, T.; Huang, Y.; Zhang, X.; Cai, Q.; Deng, X.; Yang, X. Mimicking the electrophysiological microenvironment of bone tissue using electroactive materials to promote its regeneration. J. Mater. Chem. 2020, 8, 10221–10256. [Google Scholar] [CrossRef]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.G.; Sinibaldi, E.; Ceseracciu, L.; Labardi, M.; Marino, A.; Marras, S.; De Simoni, G.; Mattoli, V.; Ciofani, G. Ultrasound-activated piezoelectric P(VDF-TrFE)/Boron Nitride nanotube composite films promote differentiation of human SaOS-2 osteoblast-like cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2421–2432. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Ricotti, L.; Moscato, S.; Bertoni, G.; Falqui, A.; Berrettini, S.; Petrini, M.; Mattoli, V.; et al. Enhancement of neurite outgrowth in neuronal-like cells following Boron Nitride nanotube- mediated stimulation. ACS Nano 2010, 4, 6267–6277. [Google Scholar] [CrossRef] [PubMed]
- Kitsara, M.; Blanquer, A.; Murillo, G.; Humblot, V.; De Bragança Vieira, S.; Nogués, C.; Ibáñez, E.; Esteve, J.; Barrios, L. Permanently hydrophilic, piezoelectric PVDF nanofibrous scaffolds promoting unaided electromechanical stimulation on osteoblasts. Nanoscale 2019, 11, 8906–8917. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.; McNamee, E.; Daniel, S. Piezoelectric nanofibers as biomaterials for bone regeneration and wound healing. Undergrad. Res. MSE 2020, 1. [Google Scholar] [CrossRef]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020, 258, 120280. [Google Scholar]
- Farahani, A.; Zarei-Hanzaki, A.; Abedi, H.R.; Daryoush, S.; Ragheb, Z.D.; Mianabadi, F.; Shahparvar, S.; Akrami, M.; Mostafavi, E.; Khanbareh, H.; et al. Silk-Based Biopolymers Promise Extensive Biomedical Applications in Tissue Engineering, Drug Delivery, and BioMEMS. J. Polym. Environ. 2023, 1–24. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef]
- Low, K.G. Remote-Activated Electrical Stimulation via Piezoelectric Scaffold System for Functional Peripheral and Central Nerve Regeneration; University of California: Riverside, CA, USA, 2017. [Google Scholar]
- Fang, J.; Izumi, C.; Iwasa, K.H. Sensitivity of prestin-based membrane motor to membrane thickness. Biophys. J. 2010, 98, 2831–2838. [Google Scholar] [CrossRef]
- Ribeiro, S.; Gomes, A.C.; Etxebarria, I.; Lanceros-Méndez, S.; Ribeiro, C. Electroactive biomaterial surface engineering effects on muscle cells differentiation. Mater. Sci. Eng. C 2018, 92, 868–874. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Xiao, J.; Desai, M.S.; Zhang, X.; Lee, S.W. Vertical self-assembly of polarized phage nanostructure for energy harvesting. Nano Lett. 2019, 19, 2661–2667. [Google Scholar] [CrossRef]
- Kim, D.; Han, S.A.; Kim, J.H.; Lee, J.H.; Kim, S.W.; Lee, S.W. Biomolecular piezoelectric materials: From amino acids to living tissues. Adv. Mater. 2020, 32, 1906989. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, A.; Atepalikhin, V.; Pakhomov, O.; Kholkin, A.L.; Tselev, A. An atomic force microscopy mode for nondestructive electromechanical studies and its application to diphenylalanine peptide nanotubes. Ultramicroscopy 2018, 185, 49–54. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, J.R.; Waigh, T.A. Electronics of peptide-and protein-based biomaterials. Adv. Colloid Interface Sci. 2021, 287, 102319. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019, e3429527. [Google Scholar]
- Ji, J.; Yang, C.; Shan, Y.; Sun, M.; Cui, X.; Xu, L.; Liang, S.; Li, T.; Fan, Y.; Luo, D.; et al. Research Trends of Piezoelectric Nanomaterials in Biomedical Engineering. Adv. NanoBiomed. Res. 2023, 3, 2200088. [Google Scholar] [CrossRef]
- Lay, R.; Deijs, G.S.; Malmström, J. The intrinsic piezoelectric properties of materials–a review with a focus on biological materials. RSC Adv. 2021, 11, 30657–30673. [Google Scholar] [CrossRef]
- Cao, L.; Qiu, X.; Jiao, Q.; Zhao, P.; Li, J.; Wei, Y. Polysaccharides and proteins-based nanogenerator for energy harvesting and sensing: A review. Int. J. Biol. Macromol. 2021, 173, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, M.B.; Leppik, L.; Costa Oliveira, K.M.; Barker, J.H. Role of bioelectricity during cell proliferation in dfferent cell types. Front. Bioeng. Biotechnol. 2020, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, X.; Dai, H.; San, Z.; Wang, F.; Ren, L.; Li, G. Polycaprolactone/Calcium Sulfate Whisker/Barium Titanate piezoelectric ternary composites for tissue reconstruction. Adv. Compos. Lett. 2020, 29, 2633366X1989792. [Google Scholar] [CrossRef]
- Hart, N.H.; Newton, R.U.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy physiology and measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347–371. [Google Scholar]
- Lees, D.; Partington, P. Articular cartilage. Orthop. Trauma 2016, 30, 265–272. [Google Scholar] [CrossRef]
- Arinzeh, T.L.; Weber, N.; Jaffe, M. Electrospun Electroactive Polymers for Regenerative Medicine Applications. U.S. Patent 10,052,412, 2018. [Google Scholar]
- More, N.; Kapusetti, G. Piezoelectric material—A promising approach for bone and cartilage regeneration. Med. Hypotheses 2017, 108, 10–16. [Google Scholar] [CrossRef]
- Williams, J.P.; Micoli, K.; McDonald, J.M. Calmodulin—An often ignored signal in osteoclasts. Ann. NY Acad. Sci. 2010, 1192, 358–364. [Google Scholar] [CrossRef]
- Regling, G. Intra-articular measurement of resting synovial pO2 (oxygen partial pressure of synovial fluid)-a new point of intersection for clinical research in the areas of arthrosis and pain. In Wolff’s Law and Connective Tissue Regulation: Modern Interdisciplinary Comments on Wolff’s Law of Connective Tissue Regulation and Rational Understanding of Common Clinical Problems; De Gruyter: New York, NY, USA, 1992; pp. 299–320. [Google Scholar]
- Kapat, K.; Shubhra, Q.T.H.; Zhou, M.; Leeuwenburgh, S. Piezoelectric nano- biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng. Part A 2009, 15, 3605–3619. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Collins, G.; Livingston Arinzeh, T. Neurite extension of primary neurons on electrospun piezoelectric scaffolds. Acta Biomater. 2011, 7, 3877–3886. [Google Scholar] [CrossRef]
- Royo-Gascon, N.; Wininger, M.; Scheinbeim, J.I.; Firestein, B.L.; Craelius, W. Piezoelectric substrates promote neurite growth in rat spinal cord neurons. Ann. Biomed. Eng. 2013, 41, 112–122. [Google Scholar] [CrossRef]
- Reis, J.; Frias, C.; Canto e Castro, C.; Botelho, M.L.; Marques, A.T.; Simões, J.A.O.; Capela e Silva, F.; Potes, J. A new piezoelectric actuator induces bone formation in vivo: A preliminary study. J. Biomed. Biotechnol. 2012, 2012, 613403. [Google Scholar] [CrossRef]
- Zhang, P. Sensors and Actuators. In Advanced Industrial Control Technology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 73–116. [Google Scholar]
- Spanner, K.; Koc, B. Piezoelectric motors, an overview. Actuators 2016, 5, 6. [Google Scholar] [CrossRef]
- Lendlein, A.; Gould, O.E.C. Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Jia, H.; Gu, S.-Y.; Chang, K. 3D printed self-expandable vascular stents from biodegradable shape memory polymer. Adv. Polym. Technol. 2018, 37, 3222–3228. [Google Scholar] [CrossRef]
- Li, G.; Fei, G.; Xia, H.; Han, J.; Zhao, Y. Spatial and temporal control of shape memory polymers and simultaneous drug release using high intensity focused ultrasound. J. Mater. Chem. 2012, 22, 7692. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Badami, V.; Ahuja, B. Biosmart materials: Breaking new ground in dentistry. Sci. World J. 2014, 2014, 986912. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Saigal, A.; Fonte, M. Solid, shape recovered “Bulk” Nitinol: Part I—Tension– compression asymmetry. Mater. Sci. Eng. A 2011, 528, 5536–5550. [Google Scholar] [CrossRef]
- Sampath Kumar, T.S. Physical and Chemical Characterization of Biomaterials. In Characterization of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2013; pp. 11–47. [Google Scholar]
- Huang, W.M.; Zhao, Y.; Wang, C.C.; Ding, Z.; Purnawali, H.; Tang, C.; Zhang, J.L. Thermo/chemo-responsive shape memory effect in polymers: A sketch of working mechanisms fundamentals optimization. J. Polym. Res. 2012, 19, 9952. [Google Scholar] [CrossRef]
- Iqbal, D.; Samiullah, M. Photo-responsive shape-memory and shape-changing liquid-crystal polymer networks. Materials 2013, 6, 116–142. [Google Scholar] [CrossRef]
- Yang, L.; Lou, J.; Yuan, J.; Deng, J. A review of shape memory polymers based on the intrinsic structures of their responsive switches. RSC Adv. 2021, 11, 28838–28850. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, B.; Sun, B.; Zhang, X.; Dong, Z.; Liu, Q.; Cui, T. Effect of Sm doping on the microstructure, mechanical properties and shape memory effect of Cu-13.0Al-4.0Ni Alloy. Materials 2021, 14, 4007. [Google Scholar] [CrossRef]
- Adiguzel, O. The role of twinned and detwinned structures on memory behaviour of shape memory alloys. Adv. Mater. Res. 2015, 1105, 78–82. [Google Scholar] [CrossRef]
- Dai, K.; Ning, C. Shape Memory Alloys and Their Medical Applications. In Biomechanics and Biomaterials in Orthopedics; Poitout, D.G., Ed.; Springer: London, UK, 2016; pp. 187–195. [Google Scholar]
- Smrke, D.; Roman, P.; Veselko, M.; Gubi, B. Treatment of Bone Defects—Allogenic Platelet Gel and Autologous Bone Technique. In Regenerative Medicine and Tissue Engineering; Andrades, J.A., Ed.; InTech: Houston, TX, USA, 2013. [Google Scholar]
- Zhang, D.; George, O.J.; Petersen, K.M.; Jimenez-Vergara, A.C.; Hahn, M.S.; Grunlan, M.A. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014, 10, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xia, H.; Teramoto, A.; Ni, Q.-Q. Fabrication and characterization of shape memory polyurethane porous scaffold for bone tissue engineer-ing: Shape memory polyurethane prous scaffold. J. Biomed. Mater. Res. A 2017, 105, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Wang, X.; Yuan, H.; Lou, X.; Zhao, Q.; Zhang, Y. HAp incorporated ultrafine polymeric fibers with shape memory effect for potential use in bone screw hole healing. J. Mater. Chem. B 2016, 4, 5308–5320. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.G.; Scoggins, C.R.; McMasters, K.M. Laparoscopic hepatic lobectomy: Advantages of a minimally invasive approach. J. Am. Coll. Surg. 2010, 210, 627–634. [Google Scholar] [CrossRef]
- Mandrycky, C.; Phong, K.; Zheng, Y. Tissue engineering toward organ-specific regeneration and disease modeling. MRS Commun. 2017, 7, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, V.; Maroufi, N.F.; Saghati, S.; Asadi, N.; Darabi, M.; Ahmad, S.N.S.; Hosseinkhani, H.; Rahbarghazi, R. Current progress in hepatic tissue regeneration by tissue engineering. J. Transl. Med. 2019, 17, 383. [Google Scholar] [CrossRef]
- Han, X.-J.; Dong, Z.-Q.; Fan, M.-M.; Liu, Y.; Li, J.-H.; Wang, Y.-F.; Yuan, Q.-J.; Li, B.-J.; Zhang, S. pH-induced shape-memory polymers. Macromol. Rapid Commun. 2012, 33, 1055–1060. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. The alkaline diet: Is there evidence that an alkaline pH diet benefits health? J. Environ. Public Health 2012, 2012, 727630. [Google Scholar] [CrossRef]
- Wang, T.X.; Chen, H.M.; Salvekar, A.V.; Lim, J.; Chen, Y.; Xiao, R.; Huang, W.M. Vitrimer-like shape memory polymers: Characterization and applications in reshaping and manufacturing. Polymers 2020, 12, 2330. [Google Scholar] [CrossRef]
- Song, Q.; Chen, H.; Zhou, S.; Zhao, K.; Wang, B.; Hu, P. Thermo- and pH- sensitive shape memory polyurethane containing carboxyl groups. Polym. Chem. 2016, 7, 1739–1746. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, D.; Wang, W.; Liu, Y.; Zhou, S. pH-responsive shape memory poly(ethylene glycol)–poly(ε-caprolactone)-based polyurethane/cellulose nanocrystals nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 12988–12999. [Google Scholar] [CrossRef]
- Tseng, L.-F.; Mather, P.T.; Henderson, J.H. Shape-memory-actuated change in scaffold fiber alignment directs stem cell morphology. Acta Biomater. 2013, 9, 8790–8801. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Prabhakaran, M.P.; Chan, B.Q.Y.; Liow, S.S.; Ramakrishna, S.; Loh, X.J. Biocompatible electrically conductive nanofibers from inorganic- organic shape memory polymers. Colloids Surf. B Biointerfaces 2016, 148, 557–565. [Google Scholar] [CrossRef]
- Zare, M.; Parvin, N.; Prabhakaran, M.P.; Mohandesi, J.A.; Ramakrishna, S. Highly porous 3D sponge-like shape memory polymer for tissue engineering application with remote actuation potential. Compos. Sci. Technol. 2019, 184, 107874. [Google Scholar] [CrossRef]
- Cera, L.; Gonzalez, G.M.; Liu, Q.; Choi, S.; Chantre, C.O.; Lee, J.; Gabardi, R.; Choi, M.C.; Shin, K.; Parker, K.K. A bioinspired and hierarchically structured shape-memory material. Nat. Mater. 2021, 20, 242–249. [Google Scholar] [CrossRef]
- Khoury, L.R.; Popa, I. Chemical unfolding of protein domains induces shape change in programmed protein hydrogels. Nat. Commun. 2019, 10, 5439. [Google Scholar] [CrossRef]
- Sahajpal, K.; Shekhar, S.; Kumar, A.; Sharma, B.; Meena, M.K.; Bhagi, A.K.; Sharma, S. Dynamic protein and polypeptide hydrogels based on Schiff base co-assembly for biomedicine. J. Mater. Chem. B 2022, 10, 3173–3198. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels- Synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Parhi Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [CrossRef] [PubMed]
- George, J.; Hsu, C.-C.; Nguyen, L.T.B.; Ye, H.; Cui, Z. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol. Adv. 2020, 42, 107370. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Polymeric hydrogels as technology platform for drug delivery applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-healing hydrogels: The next paradigm shift in tissue engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef]
- Mishra, S.B.; Mishra, A.K. Polymeric Hydrogels: A Review of Recent Developments. In Polymeric Hydrogels as Smart Biomaterials, Springer Series on Polymer and Composite Materials; Kalia, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–17. [Google Scholar]
- Drozdov, A.D. Equilibrium swelling of multi-stimuli-responsive superabsorbent hydrogels. Mech. Soft Mater. 2021, 3, 1. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Echeverria, C.; Fernandes, S.; Godinho, M.; Borges, J.; Soares, P. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Zeng, L.; Wu, Z.L.; Guo, H.; Hourdet, D. Stimuli-responsive toughening of hydrogels. Chem. Mater. 2021, 33, 7633–7656. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Tendulkar, G.; Chen, T.; Ehnert, S.; Kaps, H.-P.; Nüssler, A.K. Intervertebral disc nucleus repair: Hype or hope? Int. J. Mol. Sci. 2019, 20, 3622. [Google Scholar] [CrossRef] [PubMed]
- Manjua, A.C.; Alves, V.D.; Crespo, J.G.; Portugal, C.A.M. Magnetic responsive PVA hydrogels for remote modulation of protein sorption. ACS Appl. Mater. Interfaces 2019, 11, 21239–21249. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, H. Interaction of Switchable Biomaterials Surfaces with Proteins. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 167–188. [Google Scholar]
- Xu, R.; Ma, S.; Lin, P.; Yu, B.; Zhou, F.; Liu, W. High strength astringent hydrogels using protein as the building block for physically cross-linked multi-network. ACS Appl. Mater. Interfaces 2017, 10, 7593–7601. [Google Scholar] [CrossRef] [PubMed]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent trends in peptide and protein-based hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef]
- Cai, Z.; Luck, L.A.; Punihaole, D.; Madura, J.D.; Asher, S.A. Photonic crystal protein hydrogel sensor materials enabled by conformationally induced volume phase transition. Chem. Sci. 2016, 7, 4557–4562. [Google Scholar] [CrossRef]
- Nakamura, H.; Lee, A.A.; Afshar, A.S.; Watanabe, S.; Rho, E.; Razavi, S.; Suarez, A.; Lin, Y.-C.; Tanigawa, M.; Huang, B.; et al. Intracellular production of hydrogels and synthetic RNA granules by multivalent molecular interactions. Nat. Mater. 2018, 17, 79–89. [Google Scholar] [CrossRef]
- Ding, C.; Yang, Q.; Tian, M.; Guo, C.; Deng, F.; Dang, Y.; Zhang, M. Novel collagen-based hydrogels with injectable, self-healing, wound-healing properties via a dynamic crosslinking interaction. Polym. Int. 2020, 69, 858–866. [Google Scholar] [CrossRef]
- Resmi, R.; Parvathy, J.; John, A.; Joseph, R. Injectable self-crosslinking hydrogels for meniscal repair: A study with oxidized alginate and gelatin. Carbohydr. Polym. 2020, 234, 115902. [Google Scholar] [CrossRef]
- Mao, Q.; Hoffmann, O.; Yu, K.; Lu, F.; Lan, G.; Dai, F.; Shang, S.; Xie, R. Self-contracting oxidized starch/gelatin hydrogel for noninvasive wound closure and wound healing. Mater. Des. 2020, 194, 108916. [Google Scholar] [CrossRef]
- Reidy, E.; Leonard, N.A.; Treacy, O.; Ryan, A.E. A 3D view of colorectal cancer models in predicting therapeutic responses and resistance. Cancers 2021, 13, 227. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation characterization applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B. Introduction to Polymeric Gels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–27. [Google Scholar]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Temperature-Responsive Membranes. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–113. [Google Scholar]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018, 66, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Deng, F.; Peng, Y.; Chen, H.; Gao, Y.; Li, H. Redox- and pH- responsive polymer gels with reversible sol–gel transitions and self-healing properties. RSC Adv. 2014, 4, 47361–47367. [Google Scholar] [CrossRef]
- Higgs, P.L.; Ruiz-Sanchez, A.J.; Dalmina, M.; Horrocks, B.R.; Leach, A.G.; Fulton, D.A. Enhancing the kinetics of hydrazone exchange processes: An experimental and computational study. Org. Biomol. Chem. 2019, 17, 3218–3224. [Google Scholar] [CrossRef] [PubMed]
- Nevejans, S.; Ballard, N.; Miranda, J.I.; Reck, B.; Asua, J.M. The underlying mechanisms for self-healing of poly(disulfide)s. Phys. Chem. Chem. Phys. 2016, 18, 27577–27583. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Park, S.; Jin, X.; Ma, P.X. Rapid self-Integrating, injectable hydrogel for tissue complex regeneration. Adv. Healthc. Mater. 2015, 4, 1491–1495. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.; Wen, X.; Xu, Q.; Zeng, H.; Zhao, Y.; Liu, M.; Wang, Z.; Hu, X.; Wang, Y. Bio-responsive smart polymers and biomedical applications. J. Phys. Mater. 2019, 2, 032004. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Lin, C.Y.; Liu, J.C. Modular protein domains: An engineering approach toward functional biomaterials. Curr. Opin. Biotechnol. 2016, 40, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Boekhoven, J.; Dickerson, M.B.; Naik, R.R.; Stupp, S.I. Biopolymers and supramolecular polymers as biomaterials for biomedical applications. Mrs Bull. 2015, 40, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Lee, S.W. Protein-based functional nanomaterial design for bioengineering applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 69–97. [Google Scholar] [CrossRef]
- Bromley, K.M.; Morris, R.J.; Hobley, L.; Brandani, G.; Gillespie, R.M.C.; McCluskey, M.; Zachariae, U.; Marenduzzo, D.; Stanley-Wall, N.R.; MacPhee, C.E. Interfacial self-assembly of a bacterial hydrophobin. Proc. Natl. Acad. Sci. USA 2015, 112, 5419–5424. [Google Scholar] [CrossRef]
- Morris, R.J.; Bromley, K.M.; Stanley-Wall, N.; MacPhee, C.E. A phenomenological description of BslA assemblies across multiple length scales. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150131. [Google Scholar] [CrossRef]
- Singh, M.R.; Patel, S.; Singh, D. Natural Polymer-Based Hydrogels as Scaffolds for Tissue Engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–260. [Google Scholar]
- Sikdar, P.; Uddin, M.d.M.; Dip, T.M.; Islam, S.; Hoque, M.d.S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Si, Y.; Wang, L.; Wang, X.; Tang, N.; Yu, J.; Ding, B. Ultrahigh-water-content, superelastic, and shape-memory nanofiber-Assembled hydrogels exhibiting pressure-responsive conductivity. Adv. Mater. 2017, 29, 1700339. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Lin, R.; Yun, S.; Du, Y.; Wang, L.; Yao, Q.; Zannettino, A.; Zhang, H. Allogeneic primary mesenchymal stem/stromal cell aggregates within poly(N-isopropylacrylamide-co-acrylic acid) hydrogel for osteochondral regeneration. Appl. Mater. Today 2020, 18, 100487. [Google Scholar] [CrossRef]
- Qasim, M.; Arunkumar, P.; Powell, H.M.; Khan, M. Current research trends and challenges in tissue engineering for mending broken hearts. Life Sci. 2019, 229, 233–250. [Google Scholar] [CrossRef]
- Dalpé, G.; Thorogood, A.; Knoppers, B.M. A tale of two capacities: Including children and decisionally vulnerable adults in biomedical research. Front. Genet. 2019, 10, 289. [Google Scholar] [CrossRef]
- Mumme, M.; Wixmerten, A.; Miot, S.; Barbero, A.; Kaempfen, A.; Saxer, F.; Gehmert, S.; Krieg, A.; Schaefer, D.J.; Jakob, M.; et al. Tissue engineering for paediatric patients. Swiss Med. Wkly. 2019, 149, w20032. [Google Scholar] [CrossRef]




| Tissue Engineering | Scaffold Material | Effect | References |
|---|---|---|---|
| Neural tissue | Piezoelectric polymers: Polyvinylidene fluoride (PVDF) Poly[(vinylidene fluoride-co- trifluoroethylene] (PVDF-TrFE) Poly(3,4ethylenedioxythiophene) (PEDOT) Polylactic acid (PLLA) Poly(3-hydroxybutyrate-co-3- hydroxyvalerate) (PHBV) | Generate electrical signals | [23] |
| Cardiovascular tissue | Polymeric scaffold: Polyethylene terephthalate (PET) Polytetrafluoroethylene (ePTFE) Polyurethanes (PU) Polyglycolic acid (PGA) Polyesters Poly(L-lactic acid) (PLA) Poly(ε-caprolactone) (PCL) Polyhydroxyalkanoates (PHA) Polyglycerolsebacate (PGS) | Customisable material properties | [24] |
| Bone tissue | Polymeric hydrogel: Polyethylene glycol (PEG) | Release bone morphgenetic protein-2 (rhBMP-2) | [25] |
| Skin tissue | Polymeric scaffold: PCL Poly(lactide-co-glycolide) (PLGA) Polyethylene oxides (PEO) Polylactide (PLA) | Stabilise growth factor | [26] |
| Skeletal muscle tissue | Shape-memory polymers: PLA | Proliferate and differentiate C2C12 myoblast cells | [27] |
| Bone tissue | Piezoelectric polymers: (Protein) Collagen | Bone repair and regeneration | [5] |
| Types of Piezoelectric Material | Stimulation | Functions | References |
|---|---|---|---|
| Poly(vinylidenedifluoride- trifluoroethylene) (P(VDF- TrFE)) | Ultrasound | Promote cell osteogenic differentiation and proliferation, secrete ECM proteins | [42] |
| Boron nitride nanotube (BNNT) | Ultrasound | Stimulate axonal regeneration, promote neuronal electrical activity | [43] |
| PVDF nanofibrous scaffolds | Electricity | Promote unaided electromechanical stimulation on osteoblasts | [44] |
| PVDF-polycaprolactones (PCL) PVDF-multi-walled carbonnanotubes (MWCNT) | Electrical field/mechanicalforce | Heal wound, regenerate bone | [45] |
| Protein-Based Piezoelectric Material | Stimulation | Functions | References |
|---|---|---|---|
| Collagen | Electricity | Bone repair and regeneration | [5,46] |
| Silk fibroin | Mechanical force/electricity | Promotes cell growth, proliferation, and tissue regeneration | [47] |
| Types of Shape-Memory Material | Stimulation | Functions | References |
|---|---|---|---|
| Thermoplastic polyurethane (TPU) | Temperature | Control the behaviour of viable stem cells | [102] |
| Star-shaped polylactide (PLA) with aniline trimer (AT) | Electrical field | Promote C2C12 cell adhesion and proliferation, increase osteogenic differentiation of C2C12 myoblast cells | [27] |
| Poly(PCL/PDMS urethane)/carbon black nanofibres | Electrical field | Promote neuronal cell proliferation | [103] |
| Polycaprolactone dimethacrylate (PCLDMA) | Infra-red irradiation/magnetic field | Promote NIH3T3 cells proliferation | [104] |
| Keratin (protein) | Mechanical field | Protects and enables physiological functioning | [105] |
| Hydrogel Composition | Applications | Properties | References |
|---|---|---|---|
| Collagen (Collagen/dialdehyde guar gum, guar gum/borax) | Skin wound repair | Tgel = 25 °C Max G’ = 1.6 kPa | [130] |
| Gelatin (Sodium alginate dialdehyde/gelatin (15ADA20G) | Knee injury repair | Tgel = 37 °C Min tgel < 4 min Compressive strength = 295 ± 32 kPa | [131] |
| Gelatin (5.6% w/w oxidised starch/gelatin) | Wound healing | Tgel = 50 °C Elastic modulus = 36.6 kPa Compressive strength = 14.3 kPa | [132] |
| Type of Hydrogels/Examples | Composition | References |
|---|---|---|
| Homopolymeric pHEMA 2-Hydroxyethyl methacrylate (HEMA) Polyethylene glycol (PEG) | Comprised of polymer network derived from one type of species monomer | [134] |
| Copolymeric Methacrylic acid (MAA) Poly(ethylene glycol) methacrylate (PEG-PEGMA) Carboxymethyl cellulose (CMC) Polyvinylpyrrolidone (PVP) | Comprised of two or more different monomer species with at least one hydrophilic component | [135] |
| Interpenetrating polymer network (IPN) Poly(N-isopropylacrylamide) (PNIPAAM) | Comprised of more than one network that is at least partially interlaced on a molecular scale but not covalently bonded to each other | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganeson, K.; Tan Xue May, C.; Abdullah, A.A.A.; Ramakrishna, S.; Vigneswari, S. Advantages and Prospective Implications of Smart Materials in Tissue Engineering: Piezoelectric, Shape Memory, and Hydrogels. Pharmaceutics 2023, 15, 2356. https://doi.org/10.3390/pharmaceutics15092356
Ganeson K, Tan Xue May C, Abdullah AAA, Ramakrishna S, Vigneswari S. Advantages and Prospective Implications of Smart Materials in Tissue Engineering: Piezoelectric, Shape Memory, and Hydrogels. Pharmaceutics. 2023; 15(9):2356. https://doi.org/10.3390/pharmaceutics15092356
Chicago/Turabian StyleGaneson, Keisheni, Cindy Tan Xue May, Amirul Al Ashraf Abdullah, Seeram Ramakrishna, and Sevakumaran Vigneswari. 2023. "Advantages and Prospective Implications of Smart Materials in Tissue Engineering: Piezoelectric, Shape Memory, and Hydrogels" Pharmaceutics 15, no. 9: 2356. https://doi.org/10.3390/pharmaceutics15092356