Do Mixtures of Beads with Different Sizes Improve Wet Stirred Media Milling of Drug Suspensions?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.3. Theoretical Approaches
3. Results and Discussion
3.1. Breakage Kinetics, Power Consumption, Heat Generation, and Processing Issues
3.2. Further Characterization of the Milled Drug Suspensions and Particles
3.3. Microhydrodynamic (MHD) Analysis of the Impact of the Processing Conditions
3.4. Breakage Kinetics Predictions
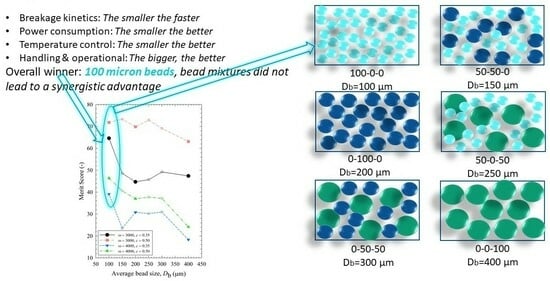
3.5. Identification of the Optimal Process–Bead Sizes Based on Merit Scores
3.6. Overall Assessment and Cost–Bead Wear Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Symbols used | |
| a | average frequency of drug particle compressions, Hz |
| c | bead loading or fractional volumetric concentration of the beads |
| d | particle size, m |
| Db | average bead size, m |
| e | restitution coefficient |
| Fbn | average max. normal force during collision of two identical elastic beads, N |
| g0 | radial distribution function at contact |
| k | breakage rate constant in Equation (8), µm1–n∙min–1 |
| K | coefficient obtained from an empirical correlation |
| n | exponent in the kinetic model |
| Nd50 | intermittent milling cycles during td50 |
| Nmc | (total) intermittent milling cycles |
| p | probability for a single drug particle to be caught between the beads |
| Pw | average stirrer power per unit volume, W/m3 |
| PSD | particle size distribution |
| R | radius, m |
| Rdiss | dissipation coefficient of the bead |
| Rdiss0 | dissipation coefficient when relative motion of the bead–liquid is absent |
| t | milling time, s |
| td50 | milling time required to attain a d50 of 0.2 µm |
| ub | average bead oscillation velocity, m/s |
| Vm | volume of the milling chamber, m3 |
| Y | Young’s modulus, Pa |
| Y* | reduced elastic modulus for the bead–drug contact, Pa |
| Greek letters | |
| αb | radius of the contact circle formed at the contact of two beads, m |
| εcoll | energy dissipation rate due to partially inelastic bead–bead collisions, W/m3 |
| εht | power spent on shear of milled suspension of the slurry at the same shear rate but calculated (measured) when no beads were present in the flow, W/m3 |
| εm | non-dimensional bead–bead gap thickness at which the lubrication force stops increasing and becomes a constant |
| εtot | total energy dissipation rate, W/m3 |
| εvisc | energy dissipation rate due to both the liquid–beads viscous friction and lubrication, W/m3 |
| η | Poisson’s ratio |
| θ | granular temperature, m2/s2 |
| µL | apparent shear viscosity, Pa·s |
| ν | frequency of single-bead oscillations, Hz |
| energy dissipation rate attributed to the deformation of drug particles per unit volume, W/m3 | |
| pseudo energy dissipation rate, J2/m6s | |
| ρ | density, kg/m3 |
| σbmax | maximum bead contact pressure at the center of the contact circle, Pa |
| σy | contact pressure in drug particle when a fully plastic condition is obtained, Pa |
| ω | stirrer (rotational) speed, rpm |
| ψ | volumetric fraction of drug particles in the drug suspension |
| Indices | |
| b | bead |
| f | final |
| L | equivalent liquid (milled drug suspension) |
| lim | limiting |
| max | maximum |
| min | minimum |
| p | drug particle |
| 10, 90 | 10% and 90% passing sizes of the cumulative PSD |
| 50 | median (50% passing size) particle size |
Appendix A
References
- Tanaka, Y.; Inkyo, M.; Yumoto, R.; Nagai, J.; Takano, M.; Nagata, S. Nanoparticulation of probucol, a poorly water-soluble drug, using a novel wet-milling process to improve in vitro dissolution and in vivo oral absorption. Drug Dev. Ind. Pharm. 2012, 38, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of drugs for bioavailability enhancement: A holistic formulation-process perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Taylor, K.M.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Surve, D.H.; Jindal, A.B. Recent advances in long-acting nanoformulations for delivery of antiretroviral drugs. J. Control. Release 2020, 324, 379–404. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: Formulation–Processing aspects and challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole nanosuspensions: Influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef]
- Bilgili, E.; Li, M.; Afolabi, A. Is the combination of cellulosic polymers and anionic surfactants a good strategy for ensuring physical stability of BCS Class II drug nanosuspensions? Pharm. Dev. Technol. 2016, 21, 499–510. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Vermant, J.; Martens, J.A.; Froyen, L.; Van Humbeeck, J.; Augustijns, P.; Van den Mooter, G. A screening study of surface stabilization during the production of drug nanocrystals. J. Pharm. Sci. 2009, 98, 2091–2103. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.-Y.; Park, C. Characteristics of polymers enabling nano-comminution of water-insoluble drugs. Int. J. Pharm. 2008, 355, 328–336. [Google Scholar] [CrossRef]
- Bilgili, E.; Guner, G. Mechanistic modeling of wet stirred media milling forproduction of drug nanosuspensions. AAPS Pharm. Sci. Technol. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Flach, F.; Breitung-Faes, S.; Kwade, A. Model based process optimization of nanosuspension preparation via wet stirred media milling. Powder Technol. 2018, 331, 146–154. [Google Scholar] [CrossRef]
- Afolabi, A.; Akinlabi, O.; Bilgili, E. Impact of process parameters on the breakage kinetics of poorly water-soluble drugs during wet stirred media milling: A microhydrodynamic view. Eur. J. Pharm. Sci. 2014, 51, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Bitterlich, A.; Laabs, C.; Busmann, E.; Grandeury, A.; Juhnke, M.; Bunjes, H.; Kwade, A. Challenges in nanogrinding of active pharmaceutical ingredients. Chem. Eng. Technol. 2014, 37, 840–846. [Google Scholar] [CrossRef]
- Li, M.; Yaragudi, N.; Afolabi, A.; Dave, R.; Bilgili, E. Sub-100 nm drug particle suspensions prepared via wet milling with low bead contamination through novel process intensification. Chem. Eng. Sci. 2015, 130, 207–220. [Google Scholar] [CrossRef]
- Juhnke, M.; Märtin, D.; John, E. Generation of wear during the production of drug nanosuspensions by wet media milling. Eur. J. Pharm. Biopharm. 2012, 81, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Guner, G.; Seetharaman, N.; Elashri, S.; Mehaj, M.; Bilgili, E. Analysis of heat generation during the production of drug nanosuspensions in a wet stirred media mill. Int. J. Pharm. 2022, 624, 122020. [Google Scholar] [CrossRef]
- Guner, G.; Elashri, S.; Mehaj, M.; Seetharaman, N.; Yao, H.F.; Clancy, D.J.; Bilgili, E. An enthalpy-balance model for timewise evolution of temperature during wet stirred media milling of drug suspensions. Pharm. Res. 2022, 39, 2065–2082. [Google Scholar] [CrossRef]
- Guner, G.; Kannan, M.; Berrios, M.; Bilgili, E. Use of bead mixtures as a novel process optimization approach to nanomilling of drug suspensions. Pharm. Res. 2021, 38, 1279–1296. [Google Scholar] [CrossRef]
- Parker, N.; Rahman, M.; Bilgili, E. Impact of media material and process parameters on breakage kinetics–energy consumption during wet media milling of drugs. Eur. J. Pharm. Biopharm. 2020, 153, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.M.; Gander, B.; Mazzotti, M. Role of milling parameters and particle stabilization on nanogrinding of drug substances of similar mechanical properties. Chem. Eng. Technol. 2011, 34, 1427–1438. [Google Scholar] [CrossRef]
- Li, M.; Alvarez, P.; Bilgili, E. A microhydrodynamic rationale for selection of bead size in preparation of drug nanosuspensions via wet stirred media milling. Int. J. Pharm. 2017, 524, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Bose, S.; Vippagunta, R.; Harmon, F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 2011, 409, 260–268. [Google Scholar] [CrossRef]
- Hennart, S.; Domingues, M.; Wildeboer, W.; Van Hee, P.; Meesters, G. Study of the process of stirred ball milling of poorly water soluble organic products using factorial design. Powder Technol. 2010, 198, 56–60. [Google Scholar] [CrossRef]
- Mende, S.; Stenger, F.; Peukert, W.; Schwedes, J. Mechanical production and stabilization of submicron particles in stirred media mills. Powder Technol. 2003, 132, 64–73. [Google Scholar] [CrossRef]
- Kawatra, S.K. Advances in Comminution; SME: Littleton, CO, USA, 2006. [Google Scholar]
- Breitung-Faes, S.; Kwade, A. Nano particle production in high-power-density mills. Chem. Eng. Res. Des. 2008, 86, 390–394. [Google Scholar] [CrossRef]
- Wang, Y.; Forssberg, E. Enhancement of energy efficiency for mechanical production of fine and ultra-fine particles in comminution. China Particuology 2007, 5, 193–201. [Google Scholar] [CrossRef]
- Azad, M.; Afolabi, A.; Bhakay, A.; Leonardi, J.; Davé, R.; Bilgili, E. Enhanced physical stabilization of fenofibrate nanosuspensions via wet co-milling with a superdisintegrant and an adsorbing polymer. Eur. J. Pharm. Biopharm. 2015, 94, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Kotake, N.; Matsumoto, K.; Sekine, K.; Gunji, S.; Kezuka, H. Effect of poly-sized and mono-sized grinding media on fine grinding of limestone in a bead mill. Int. J. Soc. Mater. 2014, 20, 147–153. [Google Scholar] [CrossRef]
- Patel, C.M.; Chakraborty, M.; Murthy, Z. Enhancement of stirred media mill performance by a new mixed media grinding strategy. J. Ind. Eng. Chem. 2014, 20, 2111–2118. [Google Scholar] [CrossRef]
- Altun, O.; Prziwara, P.; Breitung-Faes, S.; Kwade, A. Impacts of process and design conditions of dry stirred milling on the shape of product size distribution. Miner. Eng. 2021, 163, 106806. [Google Scholar] [CrossRef]
- Kataoka, M.; Masaoka, Y.; Yamazaki, Y.; Sakane, T.; Sezaki, H.; Yamashita, S. In vitro system to evaluate oral absorption of poorly water-soluble drugs: Simultaneous analysis on dissolution and permeation of drugs. Pharm. Res. 2003, 20, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Guner, G.; Yilmaz, D.; Bilgili, E. Kinetic and microhydrodynamic modeling of fenofibrate nanosuspensionproduction in a wet stirred media mill. Pharmaceutics 2021, 13, 1055. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lopez, N.; Bilgili, E. A study of the impact of polymer–surfactant in drug nanoparticle coated pharmatose composites on dissolution performance. Adv. Powder Technol. 2016, 27, 1625–1636. [Google Scholar] [CrossRef]
- Monteiro, A.; Afolabi, A.; Bilgili, E. Continuous production of drug nanoparticle suspensions via wet stirred media milling: A fresh look at the Rehbinder effect. Drug Dev. Ind. Pharm. 2013, 39, 266–283. [Google Scholar] [CrossRef]
- Guner, G.; Amjad, A.; Berrios, M.; Kannan, M.; Bilgili, E. Nanoseeded desupersaturation and dissolution tests for elucidating supersaturation maintenance in amorphous solid dispersions. Pharmaceutics 2023, 15, 450. [Google Scholar] [CrossRef] [PubMed]
- Eskin, D.; Zhupanska, O.; Hamey, R.; Moudgil, B.; Scarlett, B. Microhydrodynamic analysis of nanogrinding in stirred media mills. AIChE J. 2005, 51, 1346–1358. [Google Scholar] [CrossRef]
- Eskin, D.; Zhupanska, O.; Hamey, R.; Moudgil, B.; Scarlett, B. Microhydrodynamics of stirred media milling. Powder Technol. 2005, 156, 95–102. [Google Scholar] [CrossRef]
- Gidaspow, D. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Descriptions; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Bilgili, E.; Afolabi, A. A combined microhydrodynamics–polymer adsorption analysis for elucidation of the roles of stabilizers in wet stirred media milling. Int. J. Pharm. 2012, 439, 193–206. [Google Scholar] [CrossRef]
- Tatsumi, S.; Murayama, Y.; Hayakawa, H.; Sano, M. Experimental study on the kinetics of granular gases under microgravity. J. Fluid Mech. 2009, 641, 521–539. [Google Scholar] [CrossRef]
- Lun, C.K.K.; Savage, S.B. A simple kinetic theory for granular flow of rough, inelastic, spherical particles. J. Appl. Mech. 1987, 54, 47–53. [Google Scholar] [CrossRef]
- Guner, G.; Yilmaz, D.; Eskin, D.; Bilgili, E. Effects of bead packing limit concentration on microhydrodynamics-based prediction of breakage kinetics in wet stirred media milling. Powder Technol. 2022, 403, 117433. [Google Scholar] [CrossRef]
- Liu, G. Application of the two-fluid model with kinetic theory of granular flow in liquid–solid fluidized beds. In Granularity in Materials Science; IntechOpen: London, UK, 2018; p. 2. [Google Scholar]
- Srikar, V.T.; Turner, K.T.; Andrew Ie, T.Y.; Spearing, S.M. Structural design considerations for micromachined solid-oxide fuel cells. J. Power Sources 2004, 125, 62–69. [Google Scholar] [CrossRef]
- Maughan, M.R.; Carvajal, M.T.; Bahr, D.F. Nanomechanical testing technique for millimeter-sized and smaller molecular crystals. Int. J. Pharm. 2015, 486, 324–330. [Google Scholar] [CrossRef]
- Bhakay, A.; Merwade, M.; Bilgili, E.; Dave, R.N. Novel aspects of wet milling for the production of microsuspensions and nanosuspensions of poorly water-soluble drugs. Drug Dev. Ind. Pharm. 2011, 37, 963–976. [Google Scholar] [CrossRef]
- Azad, M.; Guner, G.; Afolabi, A.; Davé, R.; Bilgili, E. Impact of solvents during wet stirred media milling of cross-linked biopolymer suspensions. Adv. Powder Technol. 2021, 32, 4562–4575. [Google Scholar] [CrossRef]
- Knieke, C.; Azad, M.; Davé, R.; Bilgili, E. A study of the physical stability of wet media-milled fenofibrate suspensions using dynamic equilibrium curves. Chem. Eng. Res. Des. 2013, 91, 1245–1258. [Google Scholar] [CrossRef]
- Li, M.; Suriel, I.; Vekaria, J.; Proske, J.; Orbe, P.; Armani, M.; Dave, R.; Bilgili, E. Impact of dispersants on dissolution of itraconazole from drug-loaded, surfactant-free, spray-dried nanocomposites. Powder Technol. 2018, 339, 281–295. [Google Scholar] [CrossRef]
- Yue, J.; Klein, B. Effects of bead size on ultrafine grinding in a stirred bead mill. In Advances in Comminution; Kawatra, S.K., Ed.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2006; pp. 87–98. [Google Scholar]
- Mannheim, V. Empirical and scale-up modeling in stirred ball mills. Chem. Eng. Res. Des. 2011, 89, 405–409. [Google Scholar] [CrossRef]
- Nagata, S. Agitation in gas–liquid systems. In Mixing: Principles and Applications; Halsted Press: New York, NY, USA, 1975; p. 458. [Google Scholar]
- Pawar, J. Choosing the Best Media Mill for Your Wet Grinding Application. PCI Magazine RSS, 1 July 2011. [Google Scholar]
- Iddir, H.; Arastoopour, H. Modeling of multitype particle flow using the kinetic theory approach. AIChE J. 2005, 51, 1620–1632. [Google Scholar] [CrossRef]
- Fan, R.; Fox, R.O. Segregation in polydisperse fluidized beds: Validation of a multi-fluid model. Chem. Eng. Sci. 2008, 63, 272–285. [Google Scholar] [CrossRef]
- Guner, G. Microhydrodynamic, Kinetic and Thermal Modeling of Wet Media Milling for Process Optimization and Intensification. Ph.D. Thesis, New Jersey Institute of Technology, Newark, NJ, USA, 2022. [Google Scholar]
- Li, M.; Alvarez, P.; Orbe, P.; Bilgili, E. Multi-faceted characterization of wet-milled griseofulvin nanosuspensions for elucidation of aggregation state and stabilization mechanisms. AAPS Pharm. Sci. Technol. 2018, 19, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Wylie, J.J.; Koch, D.L.; Ladd, A.J.C. Rheology of suspensions with high particle inertia and moderate fluid inertia. J. Fluid Mech. 2003, 480, 95–118. [Google Scholar] [CrossRef]
- Sangani, A.S.; Mo, G.; Tsao, H.K.; Koch, D.L. Simple shear flows of dense gas-solid suspensions at finite Stokes numbers. J. Fluid Mech. 1996, 313, 309–341. [Google Scholar] [CrossRef]













| Nominal Size (µm) | d10 (µm) | d50 (µm) | d90 (µm) | Span a |
|---|---|---|---|---|
| 100 | 87 | 112 | 145 | 0.524 |
| 200 | 140 | 194 | 263 | 0.633 |
| 400 | 293 | 405 | 560 | 0.659 |
| Run No. | Stirrer Speed (rpm) | Bead Loading (-) | x100 (%) a | x200 (%) a | x400 (%) a |
|---|---|---|---|---|---|
| 1 | 3000 | 0.35 | 100 | 0 | 0 |
| 2 | 3000 | 0.35 | 50 | 50 | 0 |
| 3 | 3000 | 0.35 | 0 | 100 | 0 |
| 4 | 3000 | 0.35 | 50 | 0 | 50 |
| 5 | 3000 | 0.35 | 0 | 50 | 50 |
| 6 | 3000 | 0.35 | 0 | 0 | 100 |
| 7 | 3000 | 0.50 | 100 | 0 | 0 |
| 8 | 3000 | 0.50 | 50 | 50 | 0 |
| 9 | 3000 | 0.50 | 0 | 100 | 0 |
| 10 | 3000 | 0.50 | 50 | 0 | 50 |
| 11 | 3000 | 0.50 | 0 | 50 | 50 |
| 12 | 3000 | 0.50 | 0 | 0 | 100 |
| 13 | 4000 | 0.35 | 100 | 0 | 0 |
| 14 | 4000 | 0.35 | 50 | 50 | 0 |
| 15 | 4000 | 0.35 | 0 | 100 | 0 |
| 16 | 4000 | 0.35 | 50 | 0 | 50 |
| 17 | 4000 | 0.35 | 0 | 50 | 50 |
| 18 | 4000 | 0.35 | 0 | 0 | 100 |
| 19 | 4000 | 0.50 | 100 | 0 | 0 |
| 20 | 4000 | 0.50 | 50 | 50 | 0 |
| 21 | 4000 | 0.50 | 0 | 100 | 0 |
| 22 | 4000 | 0.50 | 50 | 0 | 50 |
| 23 | 4000 | 0.50 | 0 | 50 | 50 |
| 24 | 4000 | 0.50 | 0 | 0 | 100 |
| T1 b | 3500 | 0.43 | 100 | 0 | 0 |
| T2 b | 3500 | 0.43 | 0 | 100 | 0 |
| T3 b | 3500 | 0.43 | 0 | 0 | 100 |
| Run No. | Run Identifier | k (µm1−n/min) | n (-) | dlim (µm) | R2 | Adj R2 | SSR |
|---|---|---|---|---|---|---|---|
| 1 | 3000 0.35 100-0-0 | 0.292 | 1.62 | 0.148 | 0.992 | 0.991 | 0.066 |
| 2 | 3000 0.35 50-50-0 | 0.191 | 2.25 | 0.083 | 0.965 | 0.958 | 0.263 |
| 3 | 3000 0.35 0-100-0 | 0.214 | 2.07 | 0.107 | 0.978 | 0.974 | 0.167 |
| 4 | 3000 0.35 50-0-50 | 0.116 | 2.26 | 0.048 | 0.960 | 0.953 | 0.310 |
| 5 | 3000 0.35 0-50-50 | 0.248 | 2.01 | 0.117 | 0.976 | 0.972 | 0.183 |
| 6 | 3000 0.35 0-0-100 | 0.210 | 2.37 | 0.076 | 0.954 | 0.946 | 0.343 |
| 7 | 3000 0.50 100-0-0 | 1.54 | 1.89 | 0.130 | 0.969 | 0.964 | 0.189 |
| 8 | 3000 0.50 50-50-0 | 1.46 | 1.91 | 0.139 | 0.968 | 0.962 | 0.184 |
| 9 | 3000 0.50 0-100-0 | 1.39 | 1.92 | 0.142 | 0.973 | 0.968 | 0.158 |
| 10 | 3000 0.50 50-0-50 | 1.18 | 2.01 | 0.138 | 0.980 | 0.976 | 0.123 |
| 11 | 3000 0.50 0-50-50 | 1.28 | 1.99 | 0.141 | 0.990 | 0.988 | 0.062 |
| 12 | 3000 0.50 0-0-100 | 0.85 | 1.85 | 0.150 | 0.996 | 0.995 | 0.027 |
| 13 | 4000 0.35 100-0-0 | 1.22 | 1.18 | 0.133 | 0.890 | 0.870 | 0.777 |
| 14 | 4000 0.35 50-50-0 | 1.17 | 1.27 | 0.136 | 0.938 | 0.927 | 0.427 |
| 15 | 4000 0.35 0-100-0 | 0.571 | 1.88 | 0.140 | 0.981 | 0.977 | 0.149 |
| 16 | 4000 0.35 50-0-50 | 0.511 | 2.01 | 0.130 | 0.986 | 0.983 | 0.109 |
| 17 | 4000 0.35 0-50-50 | 0.808 | 1.76 | 0.156 | 0.997 | 0.997 | 0.018 |
| 18 | 4000 0.35 0-0-100 | 0.497 | 2.10 | 0.127 | 0.976 | 0.971 | 0.181 |
| 19 | 4000 0.50 100-0-0 | 5.10 | 2.46 | 0.119 | 0.996 | 0.995 | 0.014 |
| 20 | 4000 0.50 50-50-0 | 4.65 | 2.71 | 0.113 | 0.995 | 0.994 | 0.018 |
| 21 | 4000 0.50 0-100-0 | 3.08 | 2.64 | 0.114 | 0.994 | 0.992 | 0.025 |
| 22 | 4000 0.50 50-0-50 | 2.42 | 2.28 | 0.126 | 0.997 | 0.996 | 0.013 |
| 23 | 4000 0.50 0-50-50 | 1.87 | 2.23 | 0.128 | 0.995 | 0.994 | 0.024 |
| 24 | 4000 0.50 0-0-100 | 1.53 | 1.92 | 0.153 | 0.990 | 0.989 | 0.063 |
| Run Identifier | Approach | k (µm1−n/min) | n (-) | dlim (µm) | RMSE (µm) |
|---|---|---|---|---|---|
| 3500 0.43 100-0-0 | Direct fit | 1.45 | 1.78 | 0.144 | 0.623 |
| MHD Prediction | 1.17 | 1.91 | 0.125 | 0.604 | |
| Empirical Prediction | 1.54 | 1.89 | 0.130 | 0.668 | |
| 3500 0.43 0-100-0 | Direct fit | 1.28 | 1.88 | 0.144 | 0.049 |
| MHD Prediction | 0.953 | 1.92 | 0.125 | 0.092 | |
| Empirical Prediction | 1.39 | 1.92 | 0.142 | 0.081 | |
| 3500 0.43 0-0-100 | Direct fit | 0.814 | 2.07 | 0.144 | 0.066 |
| MHD Prediction | 0.612 | 2.01 | 0.125 | 0.183 | |
| Empirical Prediction | 0.854 | 1.85 | 0.150 | 0.120 |
| Bead Size (μm) | Price ($/kg) | Zr contamination in 6 h (μg Zr/g drug) a | Bead Wear Rate (mg/day) | Bead Wear Rate (%mass/day) | Estimated Usable Years of Beads (-) b,c | Capital Cost over 9 Years ($) b,c | Cost Savings by Full Replacement of 100 µm Beads (%) b |
|---|---|---|---|---|---|---|---|
| 100 | 549.3 | 307 | 35.0 | 0.0178 | 3.08 | 323 | – |
| 200 | 312.7 | 453 | 51.6 | 0.0263 | 2.09 | 307 | 5.12 |
| 400 | 160.2 | 832 | 94.8 | 0.0484 | 1.14 | 251 | 22.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guner, G.; Mehaj, M.; Seetharaman, N.; Elashri, S.; Yao, H.F.; Clancy, D.J.; Bilgili, E. Do Mixtures of Beads with Different Sizes Improve Wet Stirred Media Milling of Drug Suspensions? Pharmaceutics 2023, 15, 2213. https://doi.org/10.3390/pharmaceutics15092213
Guner G, Mehaj M, Seetharaman N, Elashri S, Yao HF, Clancy DJ, Bilgili E. Do Mixtures of Beads with Different Sizes Improve Wet Stirred Media Milling of Drug Suspensions? Pharmaceutics. 2023; 15(9):2213. https://doi.org/10.3390/pharmaceutics15092213
Chicago/Turabian StyleGuner, Gulenay, Mirsad Mehaj, Natasha Seetharaman, Sherif Elashri, Helen F. Yao, Donald J. Clancy, and Ecevit Bilgili. 2023. "Do Mixtures of Beads with Different Sizes Improve Wet Stirred Media Milling of Drug Suspensions?" Pharmaceutics 15, no. 9: 2213. https://doi.org/10.3390/pharmaceutics15092213