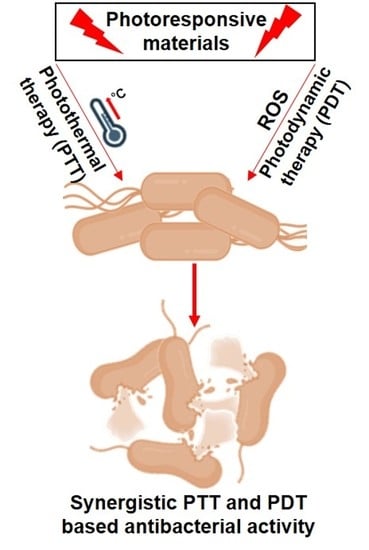
Friends against the Foe: Synergistic Photothermal and Photodynamic Therapy against Bacterial Infections
Abstract
:1. Introduction
2. Photothermal Therapy (PTT) and Photodynamic Therapy (PDT)
2.1. Photothermal Therapy (PTT)
2.1.1. Mechanism of PTT
2.1.2. Advantages of PTT
2.1.3. Limitations of PTT
2.2. Photodynamic Therapy (PDT)
2.2.1. Mechanism of PDT
2.2.2. Advantages of PDT
2.2.3. Limitations of PDT
3. Promising Aspects of Synergistic Action of PTT and PDT Combination
| Material | Light Source | Microorganisms | Ref. |
|---|---|---|---|
| BP@CCD | 660 and 808 nm | E. coli, S. aureus | [48] |
| GQDs-COS | 450 nm | E. coli, S. aureus | [49] |
| Pp4N/GNR | 660 and 808 nm | A. baumannii, MRSA | [50] |
| SiPc-F-SWNTs | 680 nm | E. coli | [51] |
| ICG loaded APTMS@SPIONs | 808 nm | E. coli, K. pneumoniae, P. aeruginosa, S. epidermidis | [52] |
| PDA@Au-HAp | 808 nm | E. coli, S. aureus | [54] |
| PATA-C4@CuS | 980 nm | Levofloxacin-resistant S. aureus, E. coli, P. aeruginosa, B. amyloliquefaciens | [55] |
| Ce6-BSA-CuS NPs | 660 and 1064 nm | E. coli, S. aureus | [56] |
| PNPG-PEG NPs | 810 nm | E. coli, S. aureus | [58] |
| CuS nanostructures | 808 nm, Simulated solar light | E. coli | [62] |
| CuS nanoparticle complex hydrogels | 808 nm | E. coli, S. aureus | [63] |
| CuS nanodots | 808 nm | β-lactamase E. coli, MRSA | [64] |
| PDA-Cur | 405 and 808 nm | E. coli, S. aureus | [65] |
| Fe3O4–Au–PDA | 808 nm | E. coli | [66] |
| BC/MoS2-CS | ≥420 nm, xenon lamp | E. coli, S. aureus | [67] |
| MoS2@Au nanorods | 808 nm, xenon lamp | E. coli | [68] |
| UCNPs@TiO2@GO-PVDF | 980 nm | E. coli, S. aureus | [69] |
| GO-tobramycin@CuS | 980 nm | Antibiotic-resistant P. aeruginosa, S. aureus | [70] |
| GO/nGO | 630 nm | E. coli, S. aureus | [71] |
| ICG-GO | 808 nm | MRSA | [72] |
| VCL/PEGDA-MNPs-MWCNTs-ZnMintPc | 630 nm | E. coli, S. aureus, C. albicans | [73] |
| nGO-BSA-AIEgen | 795 nm | AMO-resistant E. coli, S. aureus | [45] |
| BP@Te doped CDs | 808 nm | E. coli, S. aureus | [74] |
| N doped CDs@Cur | 405 and 808 nm | E. coli, S. aureus | [75] |
| Cu-CDs-C35 | 808 nm | E. coli, S. aureus | [76] |
| GQD–AgNP | 450 nm | E. coli, S. aureus | [77] |
| DMCPNs | 808 nm, White light | Ampicillin-resistant E. coli | [78] |
| PEDOT:ICG@PEG-GTA | 808 and 1064 nm | E. coli, S. aureus | [79] |
| Ti-RP/PCP/RSNO | 808 nm | MRSA | [80] |
| Ti-PDA/ICG/RGD | 808 nm | S. aureus | [81] |
| AuNRs@Cur | 405 and 808 nm | E. coli, S. aureus | [82] |
| Au@Bi2S3 | 808 nm | E. coli, S. aureus | [83] |
Synergistic PTT and PDT Agents
4. Synergistic Antibacterial Applications of PTT and PDT
4.1. Carbon-Based Combination of PTT and PDT
4.2. Polymer-Based PTT and PDT
4.3. Metal-Based Combination of PTT and PDT
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naskar, A.; Kim, K.-S. Nanomaterials as Delivery Vehicles and Components of New Strategies to Combat Bacterial Infections: Advantages and Limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M. Bacterial Infections, Antimicrobial Resistance and Antibiotic Therapy. Life 2022, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.J.; Nam, S.H.; Lee, B.J. Engineering Approaches for the Development of Antimicrobial Peptide-Based Antibiotics. Antibiotics 2022, 11, 1338. [Google Scholar] [CrossRef]
- Starr, C.G.; Wimley, W.C. Antimicrobial peptides are degraded by the cytosolic proteases of human erythrocytes. Biochim. Biophys. Acta 2017, 1859, 2319–2326. [Google Scholar] [CrossRef]
- Gong, C.; Sun, J.; Xiao, Y.; Qu, X.; Lang, M. Synthetic Mimics of Antimicrobial Peptides for the Targeted Therapy of Multidrug-Resistant Bacterial Infection. Adv. Healthc. Mater. 2021, 10, 2101244. [Google Scholar] [CrossRef]
- Mohapatra, S.; Yutao, L.; Goh, S.G.; Ng, C.; Luhua, Y.; Tran, N.H.; Gin, K.Y. Quaternary ammonium compounds of emerging concern: Classification, occurrence, fate, toxicity and antimicrobial resistance. J. Hazard Mater. 2023, 445, 130393. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, H.; Liu, X.; Zheng, Y.; Li, Z.; Li, C.; Yeung, K.W.K.; Zhu, S.; Liang, Y.; Cui, Z.; et al. Photoresponsive Materials for Antibacterial Applications. Cell Rep. Phys. Sci. 2020, 1, 100245. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Y.; Zheng, W.; Huang, Y.; Xu, F.; Bao, Z. Synergistic photothermal antibacterial therapy enabled by multifunctional nanomaterials: Progress and perspectives. Mater. Chem. Front. 2023, 7, 355–380. [Google Scholar] [CrossRef]
- Qu, Y.; Lu, K.; Zheng, Y.; Huang, C.; Wang, G.; Zhang, Y.; Yu, Q. Photothermal scaffolds/surfaces for regulation of cell behaviors. Bioact. Mater. 2021, 8, 449–477. [Google Scholar] [CrossRef]
- Alumutairi, L.; Yu, B.; Filka, M.; Nayfach, J.; Kim, M.H. Mild magnetic nanoparticle hyperthermia enhances the susceptibility of Staphylococcus aureus biofilm to antibiotics. Int. J. Hyperth. 2020, 37, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.-C.; Lin, J.-H.; Zhang, S.; Lou, C.-W.; Li, T.-T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Fan, W.; Bu, W.; Shi, J. On The Latest Three-Stage Development of Nanomedicines based on Upconversion Nanoparticles. Adv. Mater. 2016, 28, 3987–4011. [Google Scholar] [CrossRef]
- Ming, L.; Cheng, K.; Chen, Y.; Yang, R.; Chen, D. Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Med. 2021, 10, 257–268. [Google Scholar] [CrossRef]
- Huo, J.; Jia, Q.; Huang, H.; Zhang, J.; Li, P.; Dong, X.; Huang, W. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chem. Soc. Rev. 2021, 50, 8762–8789. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 10, e2001806. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.H.; Nam, J.M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef]
- Shibu, E.S.; Hamada, M.; Murase, N.; Biju, V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J. Photochem. Photobiol. C 2013, 15, 53–72. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control Release 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer-A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Kong, C.; Chen, X. Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review. Int. J. Nanomed. 2022, 17, 6427–6446. [Google Scholar] [CrossRef]
- Bera, S.; Naskar, A.; Pal, M.; Jana, S. ZnO–graphene–polyaniline nanoflowers: Solution synthesis, formation mechanism and electrochemical activity. RSC Adv. 2016, 6, 40854–40857. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.T.; Bi, Z.X.; Chen, X.; Hu, X.; Pan, W.G. A review on TiO2-x-based materials for photocatalytic CO2 reduction. Nanoscale 2022, 14, 11512–11528. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Mao, Z.; Yuan, X.; Zhang, H.; Mei, L.; Ji, X. Heterojunction Nanomedicine. Adv. Sci. 2022, 9, e2105747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, C.; Huang, R.; Ding, Y.; Ruan, C.; Shen, X.C. Mechanisms of Reactive Oxygen Species Generated by Inorganic Nanomaterials for Cancer Therapeutics. Front. Chem. 2021, 9, 630969. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Naumenko, A.P.; Marinin, A.I. Plasmonic enhancement of the antibacterial photodynamic efficiency of a zinc tetraphenylporphyrin photosensitizer/dextran graft polyacrylamide anionic copolymer/Au nanoparticles hybrid nanosystem. RSC Adv. 2021, 12, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Ye, L.; Tan, X.; Peng, J.; Zhao, L.; Zhou, Y. Silver Nanoparticle-Assisted Photodynamic Therapy for Biofilm Eradication. ACS Appl. Nano Mater. 2022, 5, 8251–8259. [Google Scholar] [CrossRef]
- Lv, H.; Liu, J.; Wang, Y.; Xia, X.; Li, Y.; Hou, W.; Li, F.; Guo, L.; Li, X. Upconversion nanoparticles and its based photodynamic therapy for antibacterial applications: A state-of-the-art review. Front. Chem. 2022, 10, 996264. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Y.; Ye, W.; Tian, L.M.; Niu, S.C.; Ming, W.H.; Zhao, J.; Ren, L.Q. Nearinfrared light triggered photodynamic and nitric oxide synergistic antibacterial nanocomposite membrane. Chem. Eng. J. 2021, 417, 128049. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 12556. [Google Scholar] [CrossRef]
- Wang, Q.; de Oliveira, E.F.; Alborzi, S.; Bastarrachea, L.J.; Tikekar, R.V. On mechanism behind UV-A light enhanced antibacterial activity of gallic acid and propyl gallate against Escherichia coli O157:H7. Sci. Rep. 2017, 7, 8325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Matsumura, Y.; Velayutham, M.; Foley, L.M.; Hitchens, T.K.; Wagner, W.R. Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 2018, 177, 98–112. [Google Scholar] [CrossRef]
- McMillan, T.J.; Leatherman, E.; Ridley, A.; Shorrocks, J.; Tobi, S.E.; Whiteside, J.R. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J. Pharm. Pharmacol. 2008, 60, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, H.; Liu, D.E.; An, J.; Gao, H. Construction of biocompatible bovine serum albumin nanoparticles composed of nano graphene oxide and AIEgen for dual-mode phototherapy bacteriostatic and bacterial tracking. J. Nanobiotechnol. 2019, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Giancarlo, G.V.D.; Janneri, L.Á.K.; Paulina, R.O.M. Antibacterial Strategies: Photodynamic and Photothermal Treatments Based on Carbon-Based Materials. In Biotechnology—Biosensors, Biomaterials and Tissue Engineering—Annual Volume 2023 [Working Title]; Intechopen: London, UK, 2022. [Google Scholar]
- Lagos, K.J.; Buzzá, H.H.; Bagnato, V.S.; Romero, M.P. Carbon-Based Materials in Photodynamic and Photothermal Therapies Applied to Tumor Destruction. Int. J. Mol. Sci. 2021, 23, 22. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, B.; Wu, F.; Zhang, Q.; Chu, X.; Ge, M.; Zhou, N.; Shen, J. Wound healing acceleration by antibacterial biodegradable black phosphorus nanosheets loaded with cationic carbon dots. J. Mater. Sci. 2021, 56, 6411–6426. [Google Scholar] [CrossRef]
- Mei, L.; Gao, X.; Shi, Y.; Cheng, C.; Shi, Z.; Jiao, M.; Cao, F.; Xu, Z.; Li, X.; Zhang, J. Augmented Graphene Quantum Dot-Light Irradiation Therapy for Bacteria-Infected Wounds. ACS Appl. Mater. Interfaces 2020, 12, 40153–40162. [Google Scholar] [CrossRef]
- Yu, Z.H.; Li, X.; Xu, F.; Hu, X.L.; Yan, J.; Kwon, N.; Chen, G.R.; Tang, T.; Dong, X.; Mai, Y.; et al. A Supramolecular-Based Dual-Wavelength Phototherapeutic Agent with Broad-Spectrum Antimicrobial Activity Against Drug-Resistant Bacteria. Angew. Chem. Int. Ed. 2020, 59, 3658–3664. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Ma, D.; Chen, J.; Guo, Q.; Han, X.; Chen, K.; Yang, H.; Huang, Y.; Peng, Y. A polyfluoroalkyl substituted phthalocyanine based supramolecular light switch for photothermal and photodynamic antibacterial activity against Escherichia coli. Chem. Commun. 2018, 54, 13279–13282. [Google Scholar] [CrossRef]
- Bilici, K.; Atac, N.; Muti, A.; Baylam, I.; Dogan, O.; Sennaroglu, A.; Can, F.; Yagci Acar, H. Broad spectrum antibacterial photodynamic and photothermal therapy achieved with indocyanine green loaded SPIONs under near infrared irradiation. Biomater. Sci. 2020, 8, 4616–4625. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, X.; Fu, L.; Zhu, J.; Fan, M.; Chen, J.; Yang, C.; Yang, G.; Wu, L.; Mao, G.; et al. Ultra-efficient Antibacterial System Based on Photodynamic Therapy and CO Gas Therapy for Synergistic Antibacterial and Ablation Biofilms. ACS Appl. Mater. Interfaces 2020, 12, 22479–22491. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomater. 2018, 77, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhao, Y.; Yu, Y.; Chen, X.; Wei, X.; Zhang, X.; Li, C. Single Continuous Near-Infrared Laser-Triggered Photodynamic and Photothermal Ablation of Antibiotic-Resistant Bacteria Using Effective Targeted Copper Sulfide Nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 30470–30479. [Google Scholar] [CrossRef]
- Gao, D.Y.; Ji, X.; Wang, J.L.; Wang, Y.T.; Li, D.L.; Liu, Y.B.; Chang, K.W.; Qu, J.L.; Zheng, J.; Yuan, Z. Engineering a protein-based nanoplatform as an antibacterial agent for light activated dual-modal photothermal and photodynamic therapy of infection in both the NIR I and II windows. J. Mater. Chem. B 2018, 6, 732–739. [Google Scholar] [CrossRef]
- Naskar, A.; Cho, H.; Kim, K.-S. Black phosphorus-based CuS nanoplatform: Near-infrared-responsive and reactive oxygen species-generating agent against environmental bacterial pathogens. J. Environ. Chem. Eng. 2022, 10, 108226. [Google Scholar] [CrossRef]
- Ghayyem, S.; Barras, A.; Faridbod, F.; Szunerits, S.; Boukherroub, R. Effective PDT/PTT dual-modal phototherapeutic killing of bacteria by using poly(N-phenylglycine) nanoparticles. Mikrochim. Acta. 2022, 189, 150. [Google Scholar] [CrossRef]
- Kwon, N.; Kim, H.; Li, X.; Yoon, J. Supramolecular agents for combination of photodynamic therapy and other treatments. Chem. Sci. 2021, 12, 7248–7268. [Google Scholar] [CrossRef]
- Shao, W.; Yang, C.; Li, F.; Wu, J.; Wang, N.; Ding, Q.; Gao, J.; Ling, D. Molecular Design of Conjugated Small Molecule Nanoparticles for Synergistically Enhanced PTT/PDT. Nanomicro Lett. 2020, 12, 147. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Yang, B.; Wang, X.; Yang, G.; Wang, X.; Jiang, Y.; Wang, T.; Jiang, J. Assembled small organic molecules for photodynamic therapy and photothermal therapy. RSC Adv. 2021, 11, 10061–10074. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.T.; Kuo, T.R. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J. Colloid Interface Sci. 2022, 607 Pt 2, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, F.; Hou, Z.; Chen, Y.; Luo, X. Injectable self-healing CuS nanoparticle complex hydrogels with antibacterial, anti-cancer, and wound healing properties. Chem. Eng. J. 2021, 409, 128224. [Google Scholar] [CrossRef]
- Qiao, Y.; Ping, Y.; Zhang, H.; Zhou, B.; Liu, F.; Yu, Y.; Xie, T.; Li, W.; Zhong, D.; Zhang, Y.; et al. Laser-Activatable CuS Nanodots to Treat Multidrug-Resistant Bacteria and Release Copper Ion to Accelerate Healing of Infected Chronic Nonhealing Wounds. ACS Appl. Mater. Interfaces 2019, 11, 3809–3822. [Google Scholar] [CrossRef] [Green Version]
- Su, R.; Yan, H.; Li, P.; Zhang, B.; Zhang, Y.; Su, W. Photo-enhanced antibacterial activity of polydopamine-curcumin nanocomposites with excellent photodynamic and photothermal abilities. Photodiagn. Photodyn. Ther. 2021, 35, 102417. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Xu, K.; Xiong, Q.; Xu, Y.; Hui, A.; Xuan, S. Fe3O4–Au–polydopamine hybrid microcapsules with photothermal–photodynamic synergistic anti-bacterial performance. CrystEngComm 2021, 23, 6610–6619. [Google Scholar] [CrossRef]
- Shen, H.; Jiang, C.; Li, W.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Synergistic Photodynamic and Photothermal Antibacterial Activity of In Situ Grown Bacterial Cellulose/MoS2-Chitosan Nanocomposite Materials with Visible Light Illumination. ACS Appl. Mater. Interfaces 2021, 13, 31193–31205. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Chung, P.F.; Krisnawati, D.I.; Rinawati, F.; Irawan, H.; Kristanto, H.; Kuo, T.R. Gold Nanorod-Decorated Metallic MoS2 Nanosheets for Synergistic Photothermal and Photodynamic Antibacterial Therapy. Nanomaterials 2021, 11, 3064. [Google Scholar] [CrossRef]
- Sun, J.; Song, L.; Fan, Y.; Tian, L.; Luan, S.; Niu, S.; Ren, L.; Ming, W.; Zhao, J. Synergistic Photodynamic and Photothermal Antibacterial Nanocomposite Membrane Triggered by Single NIR Light Source. ACS Appl. Mater. Interfaces 2019, 11, 26581–26589. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, Y.; Yu, Y.; Chen, X.; Wei, X.; Zhang, X.; Li, C. All-in-one NIR-activated nanoplatforms for enhanced bacterial biofilm eradication. Nanoscale 2018, 10, 18520–18530. [Google Scholar] [CrossRef]
- Romero, M.P.; Marangoni, V.S.; de Faria, C.G.; Leite, I.S.; Silva, C.C.C.E.; Maroneze, C.M.; Pereira-da-Silva, M.A.; Bagnato, V.S.; Inada, N.M. Graphene Oxide Mediated Broad-Spectrum Antibacterial Based on Bimodal Action of Photodynamic and Photothermal Effects. Front. Microbiol. 2020, 10, 2995. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Huang, W.; Dong, Y.; Yu, X.; Mo, A.; Peng, Q. Enhanced Antibacterial Activity of Indocyanine Green-Loaded Graphene Oxide via Synergistic Contact Killing, Photothermal and Photodynamic Therapy. J. Biomed. Nanotechnol. 2022, 18, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, C.F.; Díaz-Barrios, A.; Campaña, K.O.; Romani, E.C.; Quiroz, F.; Nardecchia, S.; Debut, A.; Vizuete, K.; Niebieskikwiat, D.; Ávila, C.E.; et al. Broad-Spectrum Antimicrobial ZnMintPc Encapsulated in Magnetic-Nanocomposites with Graphene Oxide/MWCNTs Based on Bimodal Action of Photodynamic and Photothermal Effects. Pharmaceutics 2022, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Su, Y.; Wu, S.; Shen, J. Local photothermal/photodynamic synergistic antibacterial therapy based on two-dimensional BP@CQDs triggered by single NIR light source. Photodiagn. Photodyn. Ther. 2022, 39, 102905. [Google Scholar] [CrossRef]
- Wen, F.; Li, P.; Meng, H.; Yan, H.; Huang, X.; Cui, H.; Su, W. Nitrogen-doped carbon dots/curcumin nanocomposite for combined Photodynamic/photothermal dual-mode antibacterial therapy. Photodiagn. Photodyn Ther. 2022, 39, 103033. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, P.; Wang, Y.; Sun, B.; Liu, Y.; Zhang, Q.; Feng, W.; Li, Z.; Li, K.; Zhou, N.; et al. Near-infrared carbon dot-based platform for bioimaging and photothermal/photodynamic/quaternary ammonium triple synergistic sterilization triggered by single NIR light source. Carbon 2021, 176, 126–138. [Google Scholar] [CrossRef]
- Yu, Y.; Mei, L.; Shi, Y.; Zhang, X.; Cheng, K.; Cao, F.; Zhang, L.; Xu, J.; Li, X.; Xu, Z. Ag-Conjugated graphene quantum dots with blue light-enhanced singlet oxygen generation for ternary-mode highly-efficient antimicrobial therapy. J. Mater. Chem. B 2020, 8, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, Y.; Zhao, H.; Qi, R.; Chen, Z.; Yuan, H.; Liang, H.; Wang, L. Dual-Mode Antibacterial Conjugated Polymer Nanoparticles for Photothermal and Photodynamic Therapy. Macromol. Biosci. 2020, 20, e1900301. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.; Hao, P.; Wang, Z.; Fu, L.; Ma, Z.; Zhou, J. PEDOT nanocomposites mediated dual-modal photodynamic and photothermal targeted sterilization in both NIR I and II window. Biomaterials 2015, 41, 132–140. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, B.; Zheng, Y.; Han, Y.; Chen, D.F.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; et al. Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm Infection on Bone Implant. ACS Nano 2020, 14, 8157–8170. [Google Scholar] [CrossRef]
- Yuan, Z.; Tao, B.; He, Y.; Mu, C.; Liu, G.; Zhang, J.; Liao, Q.; Liu, P.; Cai, K. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials 2019, 223, 119479. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Tang, J.; Li, P.; Su, R.; Zhong, H.; Su, W. Dual-mode antibacterial core-shell gold nanorod@mesoporous-silica/curcumin nanocomplexes for efficient photothermal and photodynamic therapy. J. Photochem. Photobiol. A 2022, 425, 113722. [Google Scholar] [CrossRef]
- Wang, W.-N.; Pei, P.; Chu, Z.-Y.; Chen, B.-J.; Qian, H.-S.; Zha, Z.-B.; Zhou, W.; Liu, T.; Shao, M.; Wang, H. Bi2S3 coated Au nanorods for enhanced photodynamic and photothermal antibacterial activities under NIR light. Chem. Eng. J. 2020, 397, 125488. [Google Scholar] [CrossRef]
- Chen, G.; Cao, Y.; Tang, Y.; Yang, X.; Liu, Y.; Huang, D.; Zhang, Y.; Li, C.; Wang, Q. Advanced Near-Infrared Light for Monitoring and Modulating the Spatiotemporal Dynamics of Cell Functions in Living Systems. Adv. Sci. 2020, 7, 1903783. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.; Kim, K.-s. Photo-Stimuli-Responsive CuS Nanomaterials as Cutting-Edge Platform Materials for Antibacterial Applications. Pharmaceutics 2022, 14, 2343. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Lin, W.; Huang, Y.; Xia, J.; Xu, J.F.; Zhang, J.; Pi, J. Advances and Potentials of Polydopamine Nanosystem in Photothermal-Based Antibacterial Infection Therapies. Front. Pharmacol. 2022, 13, 829712. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Bera, S.; Bhattacharya, R.; Saha, P.; Roy, S.S.; Sen, T.; Jana, S. Synthesis, characterization and antibacterial activity of Ag incorporated ZnO–graphene nanocomposites. RSC Adv. 2016, 6, 88751–88761. [Google Scholar] [CrossRef]
- Naskar, A.; Bera, S.; Bhattacharya, R.; Roy, S.S.; Jana, S. Effect of bovine serum albumin immobilized Au–ZnO–graphene nanocomposite on human ovarian cancer cell. J. Alloys Compd. 2018, 734, 66–74. [Google Scholar] [CrossRef]
- Aunkor, M.T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial activity of graphene oxide nanosheet against multidrug resistant superbugs isolated from infected patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef] [PubMed]
- Syama, S.; Mohanan, P.V. Comprehensive Application of Graphene: Emphasis on Biomedical Concerns. Nanomicro Lett. 2019, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.; Lee, S.; Kim, K.-s. Au-ZnO Conjugated Black Phosphorus as a Near-Infrared Light-Triggering and Recurrence-Suppressing Nanoantibiotic Platform against Staphylococcus aureus. Pharmaceutics 2021, 13, 52. [Google Scholar] [CrossRef]
- Naskar, A.; Cho, H.; Kim, K.-s. A Nanocomposite with Extracellular Vesicles from Lactobacillus paracasei as a Bioinspired Nanoantibiotic Targeting Staphylococcus aureus. Pharmaceutics 2022, 14, 2273. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Naskar, A.; Lee, S.; Kim, S.; Kim, K.-S. A New Surface Charge Neutralizing Nano-Adjuvant to Potentiate Polymyxins in Killing Mcr-1 Mediated Drug-Resistant Escherichia coli. Pharmaceutics 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Schanze, K.S. Functionalization of Water-Soluble Conjugated Polymers for Bioapplications. ACS Appl. Mater. Interfaces 2022, 14, 20506–20519. [Google Scholar] [CrossRef]
- Cui, Q.; Yuan, H.; Bao, X.; Ma, G.; Wu, M.; Xing, C. Synergistic Photodynamic and Photothermal Antibacterial Therapy Based on a Conjugated Polymer Nanoparticle-Doped Hydrogel. ACS Appl. Bio Mater. 2020, 3, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, K.; Chen, Q.; Liu, Z. Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano 2012, 6, 5605–5613. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.-S. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.-S. Black phosphorus nanomaterials as multi-potent and emerging platforms against bacterial infections. Microb. Pathog. 2019, 137, 103800. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, X.; Liao, J.; Lin, Z.; Chen, L.; Liu, D.; Zhang, T.; Li, L.; Lu, Y.; Xia, H. Synergistic Photothermal and Photodynamic Therapy for Effective Implant-Related Bacterial Infection Elimination and Biofilm Disruption Using Cu9S8 Nanoparticles. ACS Biomater. Sci. Eng. 2019, 5, 6243–6253. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naskar, A.; Kim, K.-s. Friends against the Foe: Synergistic Photothermal and Photodynamic Therapy against Bacterial Infections. Pharmaceutics 2023, 15, 1116. https://doi.org/10.3390/pharmaceutics15041116
Naskar A, Kim K-s. Friends against the Foe: Synergistic Photothermal and Photodynamic Therapy against Bacterial Infections. Pharmaceutics. 2023; 15(4):1116. https://doi.org/10.3390/pharmaceutics15041116
Chicago/Turabian StyleNaskar, Atanu, and Kwang-sun Kim. 2023. "Friends against the Foe: Synergistic Photothermal and Photodynamic Therapy against Bacterial Infections" Pharmaceutics 15, no. 4: 1116. https://doi.org/10.3390/pharmaceutics15041116