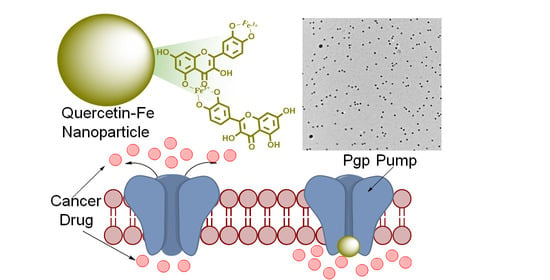
Synthesis and Characterization of Quercetin–Iron Complex Nanoparticles for Overcoming Drug Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of QFeNPs
2.3. Stability of QFeNPs
2.4. Solubility of QFeNPs
2.5. DPPH Antioxidant Activity
2.6. Characterization
2.7. P-gp Expression and Blocking Assay
3. Results and Discussion
3.1. Stability of Free Quercetin
3.2. Synthesis of QFeNPs
3.2.1. Temperature Effects
3.2.2. Q-Fe Ratio Effects
3.3. Stability of NPs
3.4. Solubility of NPs
3.5. Antioxidant Activity of QFeNPs
3.6. Inhibitory Activity of QFeNPs on P-Glycoprotein Function
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A concise review on cancer treatment methods and delivery systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. P-glycoprotein: One mechanism, many tasks and the consequences for pharmacotherapy of cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Tagde, P.; Ahmed, Z.; Khan, F.S.; et al. Multidrug resistance of cancer cells and the vital role of P-glycoprotein. Life 2022, 12, 897. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.-W. Reversal of multidrug resistance in cancer by multi-functional flavonoids. Front. Oncol. 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef]
- To, K.K.; Cho, W.C. Flavonoids overcome drug resistance to cancer chemotherapy by epigenetically modulating multiple mechanisms. Curr. Cancer Drug Targets 2021, 21, 289–305. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal complexes of flavonoids: Their synthesis, characterization and enhanced antioxidant and anticancer activities. Futur. Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yang, X.; He, J.; Cai, S.; Xiao, K.; Zhu, L. Enhanced antioxidant activity, antibacterial activity and hypoglycemic effect of luteolin by complexation with manganese (ii) and its inhibition kinetics on xanthine oxidase. RSC Adv. 2017, 7, 53385–53395. [Google Scholar] [CrossRef] [Green Version]
- Maruszewska, A.; Tarasiuk, J. Quercetin triggers induction of apoptotic and lysosomal death of sensitive and multidrug resistant leukaemia HL60 cells. Nutr. Cancer 2020, 73, 484–501. [Google Scholar] [CrossRef]
- Singh, A.; Patel, S.K.; Kumar, P.; Das, K.C.; Verma, D.; Sharma, R.; Tripathi, T.; Giri, R.; Martins, N.; Garg, N. Quercetin acts as a P-gp modulator via impeding signal transduction from nucleotide-binding domain to transmembrane domain. J. Biomol. Struct. Dyn. 2020, 40, 4507–4515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, J.; Wang, K.; Han, W.; Wang, X.; Gao, M.; Wang, Z.; Sun, Y.; Yan, H.; Zhang, H.; et al. Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur. J. Pharmacol. 2020, 881, 173185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, C.; Ma, T.; Jiang, L.; Tang, L.; Shi, T.; Zhang, S.; Zhang, L.; Zhu, P.; Li, J.; et al. Reversal effect of quercetin on multidrug resistance via FZD7/β-catenin pathway in hepatocellular carcinoma cells. Phytomedicine 2018, 43, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zohaib, B.; Li, G.; Wang, L. Effect of quercetin on P-glycoprotein expression and efflux function in liver and jejunum of rat. Acta Vet. Et Zootech. Sin. 2018, 49, 422–431. [Google Scholar]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Pinheiro, M.; Neves, A.R. Nanotechnology Innovations to Enhance the Therapeutic Efficacy of Quercetin. Nanomaterials 2021, 11, 2658. [Google Scholar] [CrossRef]
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; De Risi, C. Research Progress in the Modification of Quercetin Leading to Anticancer Agents. Molecules 2017, 22, 1270. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Ferry, D.R.; Anderson, D.; Hussain, S.A.; Young, A.M.; Cook, J.E.; Hodgkin, E.; Seymour, L.W.; Kerr, D.J. Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann. Oncol. 2001, 12, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-P.; Chen, L.-J.; Fan, L.-Y.; Tang, M.-H.; Yang, G.-L.; Yang, H.-S.; Du, X.-B.; Wang, G.-Q.; Yao, W.-X.; Zhao, Q.-M.; et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin. Cancer Res. 2006, 12, 3193–3199. [Google Scholar] [CrossRef] [Green Version]
- Zang, X.; Cheng, M.; Zhang, X.; Chen, X. Quercetin nanoformulations: A promising strategy for tumor therapy. Food Funct. 2021, 12, 6664–6681. [Google Scholar] [CrossRef]
- da Silva, W.M.B.; Pinheiro, S.D.O.; Alves, D.R.; de Menezes, J.E.S.A.; Magalhães, F.E.A.; Silva, F.C.O.; Silva, J.; Marinho, E.S.; de Morais, S.M. Synthesis of quercetin-metal complexes, in vitro and in silico anticholinesterase and antioxidant evaluation, and in vivo toxicological and anxiolitic activities. Neurotox. Res. 2019, 37, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef]
- Raza, A.; Xu, X.; Xia, L.; Xia, C.; Tang, J.; Ouyang, Z. Quercetin-iron complex: Synthesis, characterization, antioxidant, DNA binding, DNA cleavage, and antibacterial activity studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Shastrala, K.; Kalam, S.; Damerakonda, K.; Sheshagiri, S.B.B.; Kumar, H.; Guda, R.; Kasula, M.; Bedada, S.K. Synthesis, characterization, and pharmacological evaluation of some metal complexes of quercetin as P-gp inhibitors. Futur. J. Pharm. Sci. 2021, 7, 99. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, M. Studies on transition metal-quercetin complexes using electrospray ionization tandem mass spectrometry. Molecules 2015, 20, 8583–8594. [Google Scholar] [CrossRef] [Green Version]
- Papan, P.; Kantapan, J.; Sangthong, P.; Meepowpan, P.; Dechsupa, N. Iron (III)-quercetin complex: Synthesis, physicochemical characterization, and mri cell tracking toward potential applications in regenerative medicine. Contrast Media Mol. Imaging 2020, 2020, 1–22. [Google Scholar] [CrossRef]
- Bertleff-Zieschang, N.; Rahim, A.; Ju, Y.; Braunger, J.A.; Suma, T.; Dai, Y.; Pan, S.; Cavalieri, F.; Caruso, F. Biofunctional metal–phenolic films from dietary flavonoids. Chem. Commun. 2017, 53, 1068–1071. [Google Scholar] [CrossRef] [Green Version]
- Parhi, B.; Bharatiya, D.; Swain, S.K. Application of quercetin flavonoid based hybrid nanocomposites: A review. Saudi Pharm. J. 2020, 28, 1719–1732. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Azarin, S.M.; Kay, J.E.; Nessler, R.A.; Wilson, H.K.; Al-Ahmad, A.; Palecek, S.P.; Shusta, E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012, 30, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Stebbins, M.J.; Wilson, H.K.; Canfield, S.G.; Qian, T.; Palecek, S.P.; Shusta, E.V. Differentiation and characterization of human pluripotent stem cell-derived brain microvascular endothelial cells. Methods 2015, 101, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endres, L.M.; Schubert-Unkmeir, A.; Kim, B.J. Neisseria meningitidis infection of induced pluripotent stem-cell derived brain endothelial cells. J. Vis. Exp. 2020, 161, e61400. [Google Scholar] [CrossRef]
- Espinal, E.R.; Sharp, S.; Kim, B.J. Induced pluripotent stem cell (iPSC)-derived endothelial cells to study bacteri-al-brain endothelial cell interactions. Methods Mol. Biol. 2022, 2492, 73–101. [Google Scholar]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of Rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLoS ONE 2012, 7, e33253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Tuyet, P.-T. Synthesis and biological evaluation of quercetin–zinc (II) complex for anti-cancer and anti-metastasis of human bladder cancer cells. Vitr. Cell. Dev. Biol.-Anim. 2019, 55, 395–404. [Google Scholar] [CrossRef] [PubMed]
- de Castilho, T.S.; Matias, T.B.; Nicolini, K.P.; Nicolini, J. Study of interaction between metal ions and quercetin. Food Sci. Hum. Wellness 2018, 7, 215–219. [Google Scholar] [CrossRef]
- Ramešová, S.; Sokolová, R.; Degano, I.; Bulíčková, J.; Zabka, J.; Gál, M. On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef]
- Porto, I.C.C.M.; Nascimento, T.G.; Oliveira, J.M.S.; Freitas, P.H.; Haimeur, A.; França, R. Use of polyphenols as a strategy to prevent bond degradation in the dentin-resin interface. Eur. J. Oral Sci. 2018, 126, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Baranović, G.; Šegota, S. Infrared spectroscopy of flavones and flavonols. Reexamination of the hydroxyl and carbonyl vibrations in relation to the interactions of flavonoids with membrane lipids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 473–486. [Google Scholar] [CrossRef]
- Qiao, L.; Sun, Y.; Chen, R.; Fu, Y.; Zhang, W.; Li, X.; Chen, J.; Shen, Y.; Ye, X. Sonochemical effects on 14 flavonoids common in citrus: Relation to stability. PLoS ONE 2014, 9, e87766. [Google Scholar] [CrossRef] [Green Version]
- Ryan, P.; Hynes, M.J. The kinetics and mechanisms of the reactions of iron(III) with quercetin and morin. J. Inorg. Biochem. 2008, 102, 127–136. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Kalinowska, M.; Świderski, G.; Matejczyk, M.; Lewandowski, W. Spectroscopic, thermogravimetric and biological studies of Na(I), Ni(II) and Zn(II) complexes of quercetin. J. Therm. Anal. Calorim. 2016, 126, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.; Zhang, J.; Liu, Q.; Zhai, G. Sb(IIII)-quercetin complex: Synthesis, characterization and antioxidant activity. Life Sci. J. 2016, 13, 127–133. [Google Scholar]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinowska, M.; Lewandowska, H.; Pruszyński, M.; Świderski, G.; Gołębiewska, E.; Gryko, K.; Braun, J.; Borkowska, M.; Konieczna, M.; Lewandowski, W. Co(II) complex of quercetin–spectral, anti-/pro-oxidant and cytotoxic activity in HaCaT cell lines. Appl. Sci. 2021, 11, 9244. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; Mokhtarzadeh, A.; Ghareghoran, S.M.; Dehghan, G. Synthesis, Characterization and antioxidant property of quercetin-Tb(III) complex. Adv. Pharm. Bull. 2013, 4, 101–104. [Google Scholar] [CrossRef]
- Mutoh, K.; Tsukahara, S.; Mitsuhashi, J.; Katayama, K.; Sugimoto, Y. Estrogen-mediated post transcriptional down-regulation of P-glycoprotein in MDR1-transduced human breast cancer cells. Cancer Sci. 2006, 97, 1198–1204. [Google Scholar] [CrossRef]
- Bao, L.; Hazari, S.; Mehra, S.; Kaushal, D.; Moroz, K.; Dash, S. Increased Expression of P-Glycoprotein and Doxorubicin Chemoresistance of Metastatic Breast Cancer Is Regulated by miR-298. Am. J. Pathol. 2012, 180, 2490–2503. [Google Scholar] [CrossRef] [Green Version]
- Staud, F.; Ceckova, M.; Micuda, S.; Pavek, P. Expression and function of p-glycoprotein in normal tissues: Effect on phar-macokinetics. Methods Mol. Biol. 2010, 596, 199–222. [Google Scholar] [PubMed]
- Tsuji, A.; Terasaki, T.; Takabatake, Y.; Tenda, Y.; Tamai, I.; Yamashima, T.; Moritani, S.; Tsuruo, T.; Yamashita, J. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci. 1992, 51, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Lye, P.; Bloise, E.; Imperio, G.E.; Chitayat, D.; Matthews, S.G. Functional expression of multidrug-resistance (MDR) trans-porters in developing human fetal brain endothelial cells. Cells 2022, 11, 2259. [Google Scholar] [CrossRef]








| Sample | 2:1 | 1:1 | 1:2 | 1:3 | 1:4 | 1:5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dehydration (°C/%) | 112–131 | 110–131 | 118–149 | 120–155 | 116–160 | 113–124 | 159–181 | ||||
| 4% | 13% | 20% | 20% | 20% | 10% | 10% | |||||
| Q Decomposition (°C/%) | - | 312–463 | 653–715 | 299–491 | 702–747 | 302–471 | 290–442 | 258–345 | |||
| 64% | 2% | 49% | 3% | 46% | 54% | 38% | |||||
| Inorganics (°C/%) | N/A | >750 °C | 6% O | >750 °C | 8% O | >575 °C | 10% O | >500 °C | 11% O | >400 °C | 13% O |
| 15% Fe | 20% Fe | 24% Fe | 26% Fe | 28% Fe | |||||||
| H2O:Q:Fe | - | 3:1:1.1 | 5.5:1:1.8 | 6:1:2.3 | 6.2:1:2.6 | 6.6:1:3 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prestianni, L.; Espinal, E.R.; Hathcock, S.F.; Vollmuth, N.; Wang, P.; Holler, R.A.; Liu, S.; Kim, B.J.; Bao, Y. Synthesis and Characterization of Quercetin–Iron Complex Nanoparticles for Overcoming Drug Resistance. Pharmaceutics 2023, 15, 1041. https://doi.org/10.3390/pharmaceutics15041041
Prestianni L, Espinal ER, Hathcock SF, Vollmuth N, Wang P, Holler RA, Liu S, Kim BJ, Bao Y. Synthesis and Characterization of Quercetin–Iron Complex Nanoparticles for Overcoming Drug Resistance. Pharmaceutics. 2023; 15(4):1041. https://doi.org/10.3390/pharmaceutics15041041
Chicago/Turabian StylePrestianni, Lucas, Eric R. Espinal, Sarah F. Hathcock, Nadine Vollmuth, Pixiang Wang, Robert A. Holler, Shaoyang Liu, Brandon J. Kim, and Yuping Bao. 2023. "Synthesis and Characterization of Quercetin–Iron Complex Nanoparticles for Overcoming Drug Resistance" Pharmaceutics 15, no. 4: 1041. https://doi.org/10.3390/pharmaceutics15041041