3.1. Influence of Professional Skin Treatment on Bio-Physical Skin Properties
The ex vivo measurements were performed with skin probes (Corneometer and Tewameter) that are typically applied in vivo, i.e., in humans. Hence, the data obtained in this study can also be linked to in vivo studies. The skin probes were used to assess the TEWL and the skin hydration. The skin hydration measurements showed that the skin of the pig ears dries out during the experiment (
Figure 5). The skin hydration of the skin without professional skin treatment was reduced to about 50% after the first part of the skin treatment (after peeling) and was reduced to approximately 33% at the end of the experiment. The TEWL, indicating how much water is evaporating from the skin per time [
20], was reduced to 50%. In contrast to non-treated skin, professional skin treatments could significantly increase the skin hydration (
Figure 4—left). The effect was most pronounced during the treatment and after the treatment when massage was applied. The professional skin treatment without massage was found to result in a less efficient skin hydration. The effect was not expected, because a previous study showed that massage squeezes out water from the skin, which results in a reduced skin hydration. The oppose results in this study, i.e., massage increased the skin hydration, might be explained by the fact that the entire professional skin treatment in this study acts differently on the skin than an isolated massage. Isolated massage was found to squeeze the skin, which results in an increased density of the SC and a water layer on top of the SC [
6,
7]. In this study, the skin was professionally cleansed and peeled, which hydrated the skin. The next step was the massage of the skin with a cream, which was performed for some of the skin areas, whereas others were left without massage and without the application of the cream. In the case of the hydrated skin that was massaged, the massage might have resulted in an increase in skin density within the upper layers of the SC (cf.
Figure 1). In addition, it can be assumed that the cream or parts of the cream were incorporated into the skin during the massage. In this way, the water from the cream can be considered to cause a direct hydration of the skin, whereas the oil from the cream might have caused an indirect hydration due to its occlusive properties. Hence, oil from the cream that penetrated into the skin can be considered to “block” the channels in the SC through which water from the skin can evaporate. Due to the “smaller channels” within the SC, the water from deeper layers in the skin could not evaporate anymore, whereas for the skin that was not massaged, the “water channels” within the SC were left open, which allowed the water to evaporate over time. This resulted in a lower skin hydration at the end of the experiment and caused also lower TEWL values, i.e., less remaining water for evaporation within the skin (
Figure 5—right).
The data obtained from the skin probes were completed with the skin parameters obtained from the microscopic images from epifluorescence microscopy after digital image analysis (
Figure 6). During the experiment, the autofluorescence of the stratum corneum (AF-SC) increased for the non-treated skin (approx. +20%, Mann–Whitney test,
p < 0.05). An increase in AF-SC is caused by an increase in scattered light that is often caused by a decrease in the hydration of the SC [
21]. Hence, the data obtained from image analysis substantiate the data obtained from the bio-physical skin parameters. The AF-SC decreased during the professional skin treatment. The reduction is caused by the removal of sebum and bacteria, which possess a strong autofluorescence [
22]. In addition, a decrease in AF-SC might also be caused by an increase in skin hydration [
21,
23]. Hence, water within the SC reduces its optical density and therefore the measured AF-SC. The professional skin treatment with massage resulted in a slightly higher AF-SC, indicating—as mentioned above—an increase in optical density, probably due the squeezing of the skin.
The SCT is considered as an alternative surrogate for skin hydration, i.e., high SCT-values indicate high skin hydration and vice versa [
15,
16]. During the treatment, no differences in SCT were determined between non-treated and treated skin (
Figure 7—left) and also after the treatment no significant differences in SCT were found between the non-treated skin areas and the professionally treated skin with massage (
Figure 7—right). However, a slight but significant trend toward a lower SCT was found for the skin that underwent a professional skin treatment without massage. Data, therefore, also indicate that a professional skin treatment without massage results in a less hydrated SC at the end of the treatment.
Based on the data obtained in this part of the study, it can be concluded that mechanical skin treatments that are applied on the skin during a professional skin treatment increase the skin hydration. A professional skin treatment without massage is less beneficial when compared to a professional skin treatment with massage but results still in a significantly higher skin hydration when compared to skin without professional skin treatment (cf.
Figure 5, Welch test,
p < 0.001).
3.2. Influence of Mechanical Skin Treatments on Dermal Penetration Efficacy
The influence of the dermal penetration efficacy was determined for a hydrophilic AI surrogate and for a lipophilic AI surrogate, respectively. The penetration parameters assessed were the AROSA value that surrogates the total amount of penetrated AI and the MPD which indicates the penetration depth of the AI into the skin. In addition, also the SCT and the AF-SC were determined from the skin sections treated with the hydrophilic and lipophilic AI surrogates (
Figure 8 and
Figure 9).
Upon application of the AI solutions, i.e., water with SF and oil with NR, the changes in SCT due to professional treatments were more pronounced than for the skin where no AI formulations were applied (cf.
Figure 7 and
Figure 8). The SCT reducing effect of a professional skin treatment without massage was significant for the formulations with the lipophilic AI, and, also for all AI-treated samples, the skin hydrating effect of the professional skin treatment with massage was significant (
Figure 8). With this, the data are in line with the results obtained from the skin probe measurements (cf.
Figure 5). Data therefore provide further evidence and substantiate that a professional cosmetic skin treatment with massage increases skin hydration.
The autofluorescence of the stratum corneum was also assessed from the skin sections that were treated with AI. In contrast to the skin without AI treatment, the results of the AI-treated sections represent not only the AF-SC, but a sum of the AF-SC and the amount of AI that penetrated into the SC (
Figure 9). If the AI are applied during the professional skin treatment, the penetrated amount of AI in the SC is significantly less when compared to the application of AI on skin without professional skin treatment (
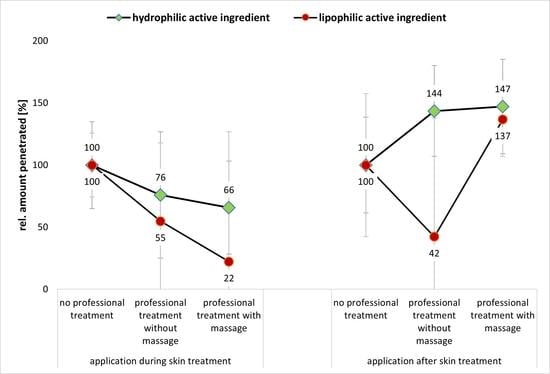
Figure 9A,B—left). The effect is less pronounced for professional skin treatments without massage and more pronounced for the professional skin treatment with massage. Additionally, the effect was more pronounced for the lipophilic AI surrogate than for the hydrophilic AI surrogate (
Figure 9A,B—left).
When the AI was applied after the professional skin treatment, the professional skin treatment with massage was found to be beneficial for the dermal penetration efficacy of the AI (
Figure 9A,B—right). However, a professional skin treatment without massage was only beneficial for the hydrophilic AI (
Figure 9A—right) but had a penetration reducing effect for the hydrophobic AI (
Figure 9B—right). Hence, data indicate that both, the professional treatment and the time point at which the AI are applied on the skin, i.e., during or after the professional skin treatment, influence the dermal penetration efficacy of AI. From the data obtained, it becomes obvious that a professional skin treatment with included massage modulates and hydrates the SC, which then enables an improved dermal penetration efficacy of AI into the SC. However, the effect can only be observed if the AI are applied onto the skin after the skin treatment is finished. Application of AI during the professional skin treatment is less effective when compared to skin without professional skin treatment. The reason for this is considered to be mainly related to the longer penetration time of the AI for the skin without professional skin treatment (cf.
Section 2.2.2). For skin without professional skin treatment, the AI were applied on the skin and remained there until the end of the experiment. In case of the skin that underwent the professional skin treatments, the formulations were applied underneath a facial mask that was removed after 30 min. The reduced amount of penetrated AI that were applied during this treatment therefore indicates that parts of the AI might have also penetrated into the mask. The even further reduced AI penetration for the skin that was massaged (
Figure 9A,B—left) indicates that the cream that was used with the massage and the pressure during the massage might have rubbed away some of the remaining AI from the skin. In fact, the application of AI during the professional skin treatment was found to be not effective when compared to the application of AI on skin without professional skin treatment. In this way, professional skin treatment without massage reduced the penetration efficacy of the hydrophilic AI surrogate into the SC to about 80% and the penetration efficacy into the SC of the lipophilic AI surrogate to about 66%. The professional skin treatment with massage reduced the penetrated amount of AI to 54% for the hydrophilic AI and to only 32% in case of the lipophilic AI (
Figure 9A,B—left).
The trends found from the AF-SC measurements were also seen for the other two penetration parameters, i.e., the AROSA values and the MPD (
Figure 10 and
Figure 11). Based on the data, it can therefore be concluded that application of AI during a professional skin treatment is not optimal because the penetration of AI is less effective when compared to the application without professional skin treatment. In addition, it is concluded that a professional cosmetic treatment should be performed with massage. This will allow for the most effective penetration of both hydrophilic and lipophilic AI if they are applied after the professional skin treatment (
Figure 9A,B—right,
Figure 10A,B—right, and
Figure 11A,B—right). Skin massage seemed to be especially important for the effective penetration of lipophilic AI (
Figure 9B—right,
Figure 10B—right, and
Figure 11B—right). A possible explanation for this observation might be the fact the massage was performed together with a cream. The skin hydration measurements already indicated that the massage with the cream increased the skin hydration when compared to skin that underwent a professional skin treatment without massage, and it was speculated that massage might have incorporated some hydrophobic compounds that close “pores” of the skin and thus prevent water loss from inner parts of the skin (cf.
Section 3.1).
Based on these considerations, it can also be speculated that the massage with the cream created a more hydrophobic SC (or skin surface/environment) than the treatment without cream and massage. When considering the results of the two abovementioned studies [
6,
7] that showed that massage can create an aqueous film on top of the skin that prevents the penetration of lipophilic AI (cf.
Figure 1), it can be assumed that also other mechanical treatments, i.e., washing, cleansing and peeling, hydrate the skin, which then results in the formation of a “water front” that hampers the penetration of lipophilic AI. In this study, the lipophilic AI was dissolved in oil. As oil does not mix with water, it is highly likely that the oil was added on top of this waterfront. The lipophilic AI surrogate—which is easily soluble in the oil but sparingly soluble in water—then preferred to remain in its preferred solvent (oil), which resulted in the observed poor penetration of the AI. In contrast, massage with the oil-containing cream can be considered to remove the waterfront on top of the skin and might even be able to incorporate some lipophilic compounds from the cream into the SC [
7]. This results in a more lipophilic skin surface into which the lipophilic AI could penetrate more easily (
Figure 9B—right,
Figure 10B—right, and
Figure 11B—right).
The data obtained for the hydrophilic AI support this theory. Here, the influence of massage is almost negligible (
Figure 10A—right, and
Figure 11A—right). The reason is that the waterfront on top of the skin will not hamper but rather accelerate the penetration of the hydrophilic AI into the skin. However, also for the hydrophilic AI, the penetration is slightly less for the professionally treated non-massaged skin when compared to the professionally treated massaged skin. The reason is the dehydration of the skin during the experiment, which reduces the penetration of the hydrophilic AI over time. Massage was found to improve the water retention of the skin (cf.
Section 3.1.). Hence, over time, good skin hydration is longer maintained for the massaged skin and thus the total penetration of the hydrophilic AI is higher when the skin is treated professionally with skin massage.
Besides the total amount of penetrated AI, it is also interesting to investigate if the AI are penetrating into the upper layers of the SC, or if they are reaching deeper layers of the viable skin. This information can be assessed by comparing the penetration depth (MPD) of the AI to the SCT. If the MPD is >SCT, the AI was able to penetrate through the SC and if the MPD is larger than the epidermis the formulation can be considered to allow for a transdermal penetration of the AI. The MPD of the hydrophilic AI, independent of the type of skin treatment, was always >150 µm (
Figure 11A). The mean thickness of the epidermis of porcine ears is in the range of about 100–110 µm and thus comparable to the thickness of the human epidermis [
24]. Hence, in this study, in all cases, a transdermal penetration of the hydrophilic AI was achieved.
In contrast, for the lipophilic AI, the MPD was about 80 µm without professional skin treatment (
Figure 11B). Hence, without professional skin treatment, the AI penetrated through the SC into the viable layers of the epidermis. Professional skin treatments with massage and application of the AI afterwards could increase the penetration depth (Mann–Whitney test,
p < 0.05). The application of the lipophilic AI onto skin after a professional treatment without massage and the application during the professional skin treatment reduced the MPD to below the SCT. Hence, in these cases, the penetration of the lipophilic AI was not deeper than the SC. Therefore, data of the study also show that mechanical treatments modulate not only the total amount of penetrated AI, but also the skin side that is reached by the AI. This fact is also important to note because the activity and efficacy of an AI depends on both the total amount of penetrated AI and the penetration depth because only AI that reach the desired target side in a sufficient dose and time can unfold their pharmacodynamic potential. Therefore, the type of skin treatment and the application time of the AI, either during or after the professional skin treatment, must be considered to be important parameters for the efficacy of AI-loaded dermal products.