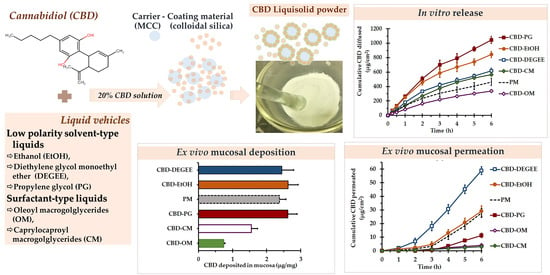
In Vitro Release, Mucosal Permeation and Deposition of Cannabidiol from Liquisolid Systems: The Influence of Liquid Vehicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CBD Solubility
2.3. Liquid Load Factor (Lf) of the CBD Solutions
2.4. Preparation of the CBD Liquisolid Powder
2.5. Evaluation of the CBD Liquisolid Powder
2.5.1. Flowability
2.5.2. Compatibility, X-ray Diffraction (XRD) and Morphological Investigations
2.5.3. In Vitro Release
2.5.4. Ex Vivo Permeation and Deposition
2.6. HPLC Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. CBD Solubility in the Liquid Vehicles
3.2. Lf of the CBD Solutions
3.3. CBD Liquisolid System Characteristics
3.3.1. Intermolecular Interaction by FTIR
3.3.2. Solid State and Morphological Characteristics
3.3.3. In Vitro Release
3.3.4. Ex Vivo Permeation and Deposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, J.K.W.; Xu, Y.; Worsley, A.; Wong, I.C.K. Oral Transmucosal Drug Delivery for Pediatric Use. Adv. Drug Deliv. Rev. 2014, 73, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical Properties of Mucus and Their Impact on Transmucosal Drug Delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Jaipakdee, N.; Tabboon, P.; Limpongsa, E. Application of a Liquisolid Technique to Cannabis Sativa Extract Compacts: Effect of Liquid Vehicles on the Dissolution Enhancement and Stability of Cannabinoids. Int. J. Pharm. 2022, 612, 121277. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Jennotte, O.; Gasparrini, Y.; Vandenbroucke, F.; Lechanteur, A.; Evrard, B. Cannabidiol Aqueous Solubility Enhancement: Comparison of Three Amorphous Formulations Strategies Using Different Type of Polymers. Int. J. Pharm. 2020, 589, 119812. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Vlad, R.-A.; Antonoaea, P.; Todoran, N.; Muntean, D.-L.; Rédai, E.M.; Silași, O.A.; Tătaru, A.; Bîrsan, M.; Imre, S.; Ciurba, A. Pharmacotechnical and Analytical Preformulation Studies for Cannabidiol Orodispersible Tablets. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2021, 29, 1029–1042. [Google Scholar] [CrossRef]
- Itin, C.; Barasch, D.; Domb, A.J.; Hoffman, A. Prolonged Oral Transmucosal Delivery of Highly Lipophilic Drug Cannabidiol. Int. J. Pharm. 2020, 581, 119276. [Google Scholar] [CrossRef]
- GW Pharmaceuticals. SATIVEX® Product Monograph Including Patient Medication Information. Available online: https://www.gwpharm.com/healthcare-professionals/sativex. (accessed on 2 February 2022).
- Damme, P.A.V.; Anastassov, G.E. Chewing Gum Compositions Comprising Cannabinoids. WO2009120080A1, 1 October 2009. [Google Scholar]
- Söpper, U.; Hoffmann, A.; Daniels, R. Mucoadhesion and Mucopenetration of Cannabidiol (CBD)-Loaded Mesoporous Carrier Systems for Buccal Drug Delivery. Sci. Pharm. 2021, 89, 35. [Google Scholar] [CrossRef]
- Temtsin-Krayz, G.; Glozman, S.; Kazhdan, P. Pharmaceutical Compositions for Transmucosal Delivery. WO2017072774A1, 4 May 2017. [Google Scholar]
- Andriotis, E.G.; Monou, P.-K.; Louka, A.; Papaefstathiou, E.; Eleftheriadis, G.K.; Fatouros, D.G. Development of Food Grade 3D Printable Ink Based on Pectin Containing Cannabidiol/Cyclodextrin Inclusion Complexes. Drug Dev. Ind. Pharm. 2020, 46, 1569–1577. [Google Scholar] [CrossRef]
- Kok, L.Y.; Bannigan, P.; Sanaee, F.; Evans, J.C.; Dunne, M.; Regenold, M.; Ahmed, L.; Dubins, D.; Allen, C. Development and Pharmacokinetic Evaluation of a Self-Nanoemulsifying Drug Delivery System for the Oral Delivery of Cannabidiol. Eur. J. Pharm. Sci. 2022, 168, 106058. [Google Scholar] [CrossRef]
- Lu, M.; Xing, H.; Jiang, J.; Chen, X.; Yang, T.; Wang, D.; Ding, P. Liquisolid Technique and Its Applications in Pharmaceutics. Asian J. Pharm. Sci. 2017, 12, 115–123. [Google Scholar] [CrossRef]
- Vraníková, B.; Gajdziok, J. Liquisolid Systems and Aspects Influencing Their Research and Development. Acta Pharm. Zagreb Croat. 2013, 63, 447–465. [Google Scholar] [CrossRef]
- Komala, D.R.; Janga, K.Y.; Jukanti, R.; Bandari, S.; Vijayagopal, M. Competence of Raloxifene Hydrochloride Loaded Liquisolid Compacts for Improved Dissolution and Intestinal Permeation. J. Drug Deliv. Sci. Technol. 2015, 30, 232–241. [Google Scholar] [CrossRef]
- Sanka, K.; Poienti, S.; Mohd, A.B.; Diwan, P.V. Improved Oral Delivery of Clonazepam through Liquisolid Powder Compact Formulations: In-Vitro and Ex-Vivo Characterization. Powder Technol. 2014, 256, 336–344. [Google Scholar] [CrossRef]
- Sharma, V.; Pathak, K. Effect of Hydrogen Bond Formation/Replacement on Solubility Characteristics, Gastric Permeation and Pharmacokinetics of Curcumin by Application of Powder Solution Technology. Acta Pharm. Sin. B 2016, 6, 600–613. [Google Scholar] [CrossRef]
- Jaipakdee, N.; Limpongsa, E.; Sripanidkulchai, B.; Piyachaturawat, P. Preparation of Curcuma Comosa Tablets Using Liquisolid Techniques: In Vitro and in Vivo Evaluation. Int. J. Pharm. 2018, 553, 157–168. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.; Awad, N. Investigating the Use of Liquisolid Compacts Technique to Minimize the Influence of PH Variations on Loratadine Release. AAPS PharmSciTech 2012, 13, 53–58. [Google Scholar] [CrossRef]
- Vraníková, B.; Gajdziok, J.; Vetchý, D. Determination of Flowable Liquid Retention Potential of Aluminometasilicate Carrier for Liquisolid Systems Preparation. Pharm. Dev. Technol. 2015, 20, 839–844. [Google Scholar] [CrossRef]
- Aodah, A.; Rawas-Qalaji, M.; Bafail, R.; Rawas-Qalaji, M. Effect of Fast-Disintegrating Tablets’ Characteristics on the Sublingual Permeability of Atropine Sulfate for the Potential Treatment of Organophosphates Toxicity. AAPS PharmSciTech 2019, 20, 229. [Google Scholar] [CrossRef]
- Sheu, M.-T.; Hsieh, C.-M.; Chen, R.-N.; Chou, P.-Y.; Ho, H.-O. Rapid-Onset Sildenafil Sublingual Drug Delivery Systems: In Vitro Evaluation and In Vivo Pharmacokinetic Studies in Rabbits. J. Pharm. Sci. 2016, 105, 2774–2781. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Soe, M.T.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. Modified Glutinous Rice Starch-Chitosan Composite Films for Buccal Delivery of Hydrophilic Drug. Carbohydr. Polym. 2020, 245, 116556. [Google Scholar] [CrossRef]
- Jaipakdee, N.; Pongjanyakul, T.; Limpongsa, E. Preparation and characterization of poly (vinyl alcohol)-poly (vinyl pyrrolidone) mucoadhesive buccal patches for delivery of lidocaine HCl. Int. J. Appl. Pharm. 2018, 10, 115–123. [Google Scholar] [CrossRef]
- Majid, H.; Puzik, A.; Maier, T.; Merk, R.; Bartel, A.; Mueller, H.-C.; Burckhardt, B.B. Formulation Development of Sublingual Cyclobenzaprine Tablets Empowered by Standardized and Physiologically Relevant Ex Vivo Permeation Studies. Pharmaceutics 2021, 13, 1409. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.M.; Sabri, A.; Fischer, P.M.; Barrett, D.A.; Constantinescu, C.S.; Gershkovich, P. Development of a Simple and Sensitive HPLC–UV Method for the Simultaneous Determination of Cannabidiol and Δ9-Tetrahydrocannabinol in Rat Plasma. J. Pharm. Biomed. Anal. 2015, 114, 145–151. [Google Scholar] [CrossRef]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy: In Manufacture, Formulation and Clinical Use, 6th ed.; Pharmaceutical press: London, UK, 2016; ISBN 978-0-85711-174-6. [Google Scholar]
- Kim, C. Advanced Pharmaceutics: Physiochemical Principles; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-8493-1729-3. [Google Scholar]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Handbook of Pharmaceutical Excipients: Edited by Raymond C. Rowe, Paul J. Sheskey, Marian E. Quinn, Rowe, R.C.; Sheskey, P.J.; Owen, S.C.; American Pharmacists Association (Eds.) , 6th ed.; APhA/Pharmaceutical Press: London, UK; Chicago, IL, USA, 2009; ISBN 978-1-58212-135-2. [Google Scholar]
- Suzuki, T.; Ebert, R.-U.; Schüürmann, G. Development of Both Linear and Nonlinear Methods To Predict the Liquid Viscosity at 20 °C of Organic Compounds. J. Chem. Inf. Comput. Sci. 1997, 37, 1122–1128. [Google Scholar] [CrossRef]
- Elkordy, A.A.; Essa, E.A.; Dhuppad, S.; Jammigumpula, P. Liquisolid Technique to Enhance and to Sustain Griseofulvin Dissolution: Effect of Choice of Non-Volatile Liquid Vehicles. Int. J. Pharm. 2012, 434, 122–132. [Google Scholar] [CrossRef]
- Tiong, N.; Elkordy, A.A. Effects of Liquisolid Formulations on Dissolution of Naproxen. Eur. J. Pharm. Biopharm. 2009, 73, 373–384. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. The United States Pharmacopeia: The National Formulary; United States Pharmacopeial: Rockville, MD, USA, 2016; ISBN 978-1-936424-60-3. [Google Scholar]
- Lane, M.E. Skin Penetration Enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Sohi, H.; Ahuja, A.; Ahmad, F.J.; Khar, R.K. Critical Evaluation of Permeation Enhancers for Oral Mucosal Drug Delivery. Drug Dev. Ind. Pharm. 2010, 36, 254–282. [Google Scholar] [CrossRef]
- Strickley, R.G. Solubilizing Excipients in Oral and Injectable Formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef]
- Khanfar, M.; Sheikh Salem, M.; Hawari, R. Formulation Factors Affecting the Release of Ezetimibe from Different Liquisolid Compacts. Pharm. Dev. Technol. 2013, 18, 417–427. [Google Scholar] [CrossRef]
- Vraníková, B.; Niederquell, A.; Ditzinger, F.; Šklubalová, Z.; Kuentz, M. Mechanistic Aspects of Drug Loading in Liquisolid Systems with Hydrophilic Lipid-Based Mixtures. Int. J. Pharm. 2020, 578, 119099. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Sun, Y.; Freeman, K.; Mchenry, M.A.; Wang, C.; Guo, M. Enhanced Stability and Oral Bioavailability of Cannabidiol in Zein and Whey Protein Composite Nanoparticles by a Modified Anti-Solvent Approach. Foods 2022, 11, 376. [Google Scholar] [CrossRef]
- Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 6th ed.; Martin, M.N.; Sinko, P.J.; Singh, Y. (Eds.) 50th anniversary ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2011; ISBN 978-0-7817-9766-5. [Google Scholar]
- Casiraghi, A.; Musazzi, U.M.; Centin, G.; Franzè, S.; Minghetti, P. Topical Administration of Cannabidiol: Influence of Vehicle-Related Aspects on Skin Permeation Process. Pharmaceuticals 2020, 13, 337. [Google Scholar] [CrossRef]
- Ng, S.-F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a Static Franz Diffusion Cell System for In Vitro Permeation Studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef]
- Adibkia, K.; Shokri, J.; Barzegar-Jalali, M.; Solduzian, M.; Javadzadeh, Y. Effect of Solvent Type on Retardation Properties of Diltiazem HCl Form Liquisolid Tablets. Colloids Surf. B Biointerfaces 2014, 113, 10–14. [Google Scholar] [CrossRef]
- Fahmy, R.; Kassem, M. Enhancement of Famotidine Dissolution Rate through Liquisolid Tablets Formulation: In Vitro and in Vivo Evaluation. Eur. J. Pharm. Biopharm. 2008, 69, 993–1003. [Google Scholar] [CrossRef]
- Van Speybroeck, M.; Williams, H.D.; Nguyen, T.-H.; Anby, M.U.; Porter, C.J.H.; Augustijns, P. Incomplete Desorption of Liquid Excipients Reduces the In Vitro and In Vivo Performance of Self-Emulsifying Drug Delivery Systems Solidified by Adsorption onto an Inorganic Mesoporous Carrier. Mol. Pharm. 2012, 9, 2750–2760. [Google Scholar] [CrossRef]
- Williams, H.D.; Speybroeck, M.V.; Augustijns, P.; Porter, C.J.H. Lipid-Based Formulations Solidified Via Adsorption onto the Mesoporous Carrier Neusilin® US2: Effect of Drug Type and Formulation Composition on In Vitro Pharmaceutical Performance. J. Pharm. Sci. 2014, 103, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Del Consuelo, I.; Jacques, Y.; Pizzolato, G.-P.; Guy, R.H.; Falson, F. Comparison of the Lipid Composition of Porcine Buccal and Esophageal Permeability Barriers. Arch. Oral Biol. 2005, 50, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Diaz del Consuelo, I.; Pizzolato, G.-P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of Pig Esophageal Mucosa as a Permeability Barrier Model for Buccal Tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Caon, T.; Simões, C.M.O. Effect of Freezing and Type of Mucosa on Ex Vivo Drug Permeability Parameters. AAPS PharmSciTech 2011, 12, 587–592. [Google Scholar] [CrossRef]
- de Araújo, J.S.M.; Volpato, M.C.; Muniz, B.V.; Xavier, G.G.A.; Martinelli, C.C.M.; Lopez, R.F.V.; Groppo, F.C.; Franz-Montan, M. Resistivity Technique for the Evaluation of the Integrity of Buccal and Esophageal Epithelium Mucosa for In Vitro Permeation Studies: Swine Buccal and Esophageal Mucosa Barrier Models. Pharmaceutics 2021, 13, 643. [Google Scholar] [CrossRef]
- Squier, C.A.; Kremer, M.J. Biology of Oral Mucosa and Esophagus. J. Natl. Cancer Inst. Monogr. 2001, 2001, 7–15. [Google Scholar] [CrossRef]
- Majid, H.; Bartel, A.; Burckhardt, B.B. Predictivity of Standardized and Controlled Permeation Studies: Ex Vivo—In Vitro—In Vivo Correlation for Sublingual Absorption of Propranolol. Eur. J. Pharm. Biopharm. 2021, 169, 12–19. [Google Scholar] [CrossRef]
- Padula, C.; Pescina, S.; Nicoli, S.; Santi, P. New Insights on the Mechanism of Fatty Acids as Buccal Permeation Enhancers. Pharmaceutics 2018, 10, 201. [Google Scholar] [CrossRef]
- Limpongsa, E.; Soe, M.T.; Jaipakdee, N. Modification of Release and Penetration Behavior of Water-Soluble Active Ingredient from Ball-Milled Glutinous Starch Matrix via Carboxymethylcellulose Blending. Int. J. Biol. Macromol. 2021, 193, 2271–2280. [Google Scholar] [CrossRef]
- Sharkawy, A.; Silva, A.M.; Rodrigues, F.; Barreiro, F.; Rodrigues, A. Pickering Emulsions Stabilized with Chitosan/Collagen Peptides Nanoparticles as Green Topical Delivery Vehicles for Cannabidiol (CBD). Colloids Surf. Physicochem. Eng. Asp. 2021, 631, 127677. [Google Scholar] [CrossRef]
- Touitou, E.; Fabin, B. Altered Skin Permeation of a Highly Lipophilic Molecule: Tetrahydrocannabinol. Int. J. Pharm. 1988, 43, 17–22. [Google Scholar] [CrossRef]
- Vanti, G.; Grifoni, L.; Bergonzi, M.C.; Antiga, E.; Montefusco, F.; Caproni, M.; Bilia, A.R. Development and Optimisation of Biopharmaceutical Properties of a New Microemulgel of Cannabidiol for Locally-Acting Dermatological Delivery. Int. J. Pharm. 2021, 607, 121036. [Google Scholar] [CrossRef]
- Sattar, M.; Lane, M.E. Oral Transmucosal Delivery of Naratriptan. Int. J. Pharm. 2016, 514, 263–269. [Google Scholar] [CrossRef]
- Junaid, M.S.A.; Tijani, A.O.; Puri, A.; Banga, A.K. In Vitro Percutaneous Absorption Studies of Cannabidiol Using Human Skin: Exploring the Effect of Drug Concentration, Chemical Enhancers, and Essential Oils. Int. J. Pharm. 2022, 616, 121540. [Google Scholar] [CrossRef]
- Paudel, K.S.; Hammell, D.C.; Agu, R.U.; Valiveti, S.; Stinchcomb, A.L. Cannabidiol Bioavailability after Nasal and Transdermal Application: Effect of Permeation Enhancers. Drug Dev. Ind. Pharm. 2010, 36, 1088–1097. [Google Scholar] [CrossRef]
- Ghafourian, T.; Nokhodchi, A.; Kaialy, W. Surfactants as Penetration Enhancers for Dermal and Transdermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 207–230. ISBN 978-3-662-47038-1. [Google Scholar]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. Buccal Penetration Enhancers—How Do They Really Work? J. Control. Release 2005, 105, 1–15. [Google Scholar] [CrossRef]





| Liquid Vehicles | CBD Solubility (mg/g) | ||||
|---|---|---|---|---|---|
| Types | Dielectric Constants * | HLB ** | Viscosity (mPa⋅s) *** | Specific Gravity **** | |
| EtOH | 24.3 | N/A | 1.2 | 0.814 ± 0.002 | >1000 |
| DEGEE | 14.1 | N/A | 4.8 | 0.969 ± 0.002 | 579.46 ± 11.79 a |
| OM | N/A | 9.0 | 75–95 | 0.924 ± 0.001 | 540.31 ± 4.59 a |
| PG | 32 | N/A | 58 | 1.027 ± 0.002 | 521.04 ± 55.42 a |
| CM | N/A | 12.0 | 80–110 | 1.041 ± 0.002 | 386.77 ± 48.85 b |
| PEG | 12.5 | N/A | 105–130 | 1.120 ± 0.003 | 342.18 ± 49.90 b |
| P20 | N/A | 16.7 | 400 | 1.084 ± 0.002 | 261.73 ± 39.33 c |
| Glycerin | 40.1 | N/A | 1490 | 1.264 ± 0.003 | 0.09 ± 0.03 d |
| Deionized water | 78.5 | N/A | 1 | 0.998 ± 0.002 | <0.005 |
| Formulations | Lf * | Flow Rate (cm3/s) | Angle of Slide (°) | Angle of Repose (°) | Flowability ** |
|---|---|---|---|---|---|
| CBD–EtOH | N/A | 6.86 ± 0.37 | 29.00 ± 0.82 | 31.01 ± 0.65 | Good |
| CBD–DEGEE | 0.145 ± 0.016 | 6.31 ± 0.26 | 32.00 ± 0.82 | 33.02 ± 0.63 | Good |
| CBD–OM | 0.153 ± 0.027 | 7.04 ± 0.03 | 30.00 ± 0.01 | 31.80 ± 1.34 | Good |
| CBD–PG | 0.181 ± 0.024 | 8.99 ± 0.45 | 29.33 ± 0.47 | 33.53 ± 0.81 | Good |
| CBD–CM | 0.178 ± 0.017 | 8.54 ± 0.63 | 29.67 ± 0.47 | 33.10 ± 0.61 | Good |
| Formulations | Zero Order | Higuchi | Q6h (µg) | Diffusion Efficiency (%) | ||
|---|---|---|---|---|---|---|
| R2 | K0 (µg·cm−2·min−1) | R2 | KH (µg·cm−2⋅min−1/2) | |||
| PM system | 0.974 ± 0.012 | 71.3 ± 7.2 | 0.996 ± 0.003 | 214.6 ± 22.4 a | 930.5 ± 52.7 a | 5.63 ± 0.74 a |
| Liquisolid systems | ||||||
| CBD–EtOH | 0.951 ± 0.008 | 133.3 ± 14.5 | 0.994 ± 0.003 | 405.4 ± 43.0 b | 1696.7 ± 193.0 b | 10.51 ± 0.92 b |
| CBD–DEGEE | 0.951 ± 0.012 | 93.8 ± 7.4 | 0.993 ± 0.003 | 285.3 ± 21.8 c | 1241.3 ± 76.3 c | 7.56 ± 0.39 c |
| CBD–OM | 0.975 ± 0.015 | 55.6 ± 2.0 | 0.995 ± 0.002 | 167.0 ± 6.9 a | 669.0 ± 17.5 d | 4.05 ± 0.32 d |
| CBD–PG | 0.968 ± 0.013 | 169.6 ± 6.1 | 0.993 ± 0.004 | 511.3 ± 20.2 d | 2102.7 ± 68.8 e | 12.45 ± 0.42 e |
| CBD–CM | 0.972 ± 0.004 | 95.4 ± 3.1 | 0.994 ± 0.002 | 287.3 ± 9.2 c | 1138.4 ± 49.6 c | 6.86 ± 0.27 c |
| Formulations | Jss (µg·cm−2·h−1) | Q6h (µg) | ER |
|---|---|---|---|
| PM system | 8.09 ± 0.67 a | 55.83 ± 6.46 a | N/A |
| Liquisolid systems | |||
| CBD–EtOH | 8.10 ± 0.59 a | 58.54 ± 7.77 a | 1.05 |
| CBD–DEGEE | 13.68 ± 0.74 b | 118.38 ± 5.79 b | 2.12 |
| CBD–OM | 0.89 ± 0.11 c | 8.27 ± 0.83 c | 0.15 |
| CBD–PG | 3.65 ± 0.51 d | 22.66 ± 3.23 d | 0.41 |
| CBD–CM | 0.68 ± 0.11 c | 6.18 ± 1.42 c | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabboon, P.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. In Vitro Release, Mucosal Permeation and Deposition of Cannabidiol from Liquisolid Systems: The Influence of Liquid Vehicles. Pharmaceutics 2022, 14, 1787. https://doi.org/10.3390/pharmaceutics14091787
Tabboon P, Pongjanyakul T, Limpongsa E, Jaipakdee N. In Vitro Release, Mucosal Permeation and Deposition of Cannabidiol from Liquisolid Systems: The Influence of Liquid Vehicles. Pharmaceutics. 2022; 14(9):1787. https://doi.org/10.3390/pharmaceutics14091787
Chicago/Turabian StyleTabboon, Peera, Thaned Pongjanyakul, Ekapol Limpongsa, and Napaphak Jaipakdee. 2022. "In Vitro Release, Mucosal Permeation and Deposition of Cannabidiol from Liquisolid Systems: The Influence of Liquid Vehicles" Pharmaceutics 14, no. 9: 1787. https://doi.org/10.3390/pharmaceutics14091787