Mucosal Delivery of Cannabidiol: Influence of Vehicles and Enhancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CBD Solubility
2.3. Vehicle Uptake of Mucosa
2.4. Ex Vivo Permeation
2.4.1. CBD Solution Preparation
2.4.2. Mucosal Membrane Preparation
2.4.3. Permeation Study
2.4.4. Permeation Data Analysis
2.5. Ex Vivo Deposition
2.6. HPLC Assay
2.7. Statistical Analysis
3. Results and Discussion
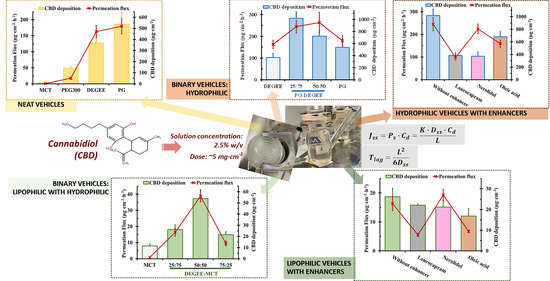
3.1. Effect of Neat Vehicles on CBD Solubility, Permeation and Deposition
3.1.1. CBD Solubility
3.1.2. CBD Permeation and Deposition
3.2. Effect of Binary Vehicles on Permeation Flux and Deposition
3.2.1. PG–DEGEE Binary Systems
3.2.2. DEGEE–MCT Binary Systems
3.3. Effect of a Combined Permeation Enhancer on Permeation Flux and Deposition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef] [PubMed]
- Jaipakdee, N.; Tabboon, P.; Limpongsa, E. Application of a Liquisolid Technique to Cannabis Sativa Extract Compacts: Effect of Liquid Vehicles on the Dissolution Enhancement and Stability of Cannabinoids. Int. J. Pharm. 2022, 612, 121277. [Google Scholar] [CrossRef]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef]
- Casiraghi, A.; Musazzi, U.M.; Centin, G.; Franzè, S.; Minghetti, P. Topical Administration of Cannabidiol: Influence of Vehicle-Related Aspects on Skin Permeation Process. Pharmaceuticals 2020, 13, 337. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Campisi, G.; Paderni, C.; Saccone, R.; Di Fede, O.; Wolff, A.; Giannola, L.I. Human Buccal Mucosa as an Innovative Site of Drug Delivery. Curr. Pharm. Des. 2010, 16, 641–652. [Google Scholar] [CrossRef]
- Di Prima, G.; Campisi, G.; De Caro, V. Amorphous Ropinirole-Loaded Mucoadhesive Buccal Film: A Potential Patient-Friendly Tool to Improve Drug Pharmacokinetic Profile and Effectiveness. J. Pers. Med. 2020, 10, 242. [Google Scholar] [CrossRef]
- Hassan, N.; Ahad, A.; Ali, M.; Ali, J. Chemical Permeation Enhancers for Transbuccal Drug Delivery. Expert Opin. Drug Deliv. 2010, 7, 97–112. [Google Scholar] [CrossRef]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. Enhancing the Buccal Mucosal Uptake and Retention of Triamcinolone Acetonide. J. Control. Release 2005, 105, 240–248. [Google Scholar] [CrossRef]
- Alomrani, A.H.; Alhazza, F.I.; AlGhamdi, K.M.; El Maghraby, G.M. Effect of Neat and Binary Vehicle Systems on the Solubility and Cutaneous Delivery of Piperine. Saudi Pharm. J. 2018, 26, 162–168. [Google Scholar] [CrossRef]
- Coutel-Egros, A.; Maitani, Y.; Veillard, M.; Machida, Y.; Nagai, T. Combined Effects of PH, Cosolvent and Penetration Enhancers on the in Vitro Buccal Absorption of Propranolol through Excised Hamster Cheek Pouch. Int. J. Pharm. 1992, 84, 117–128. [Google Scholar] [CrossRef]
- Kung, C.-P.; Zhang, Y.; Sil, B.C.; Hadgraft, J.; Lane, M.E.; Patel, B.; McCulloch, R. Investigation of Binary and Ternary Solvent Systems for Dermal Delivery of Methadone. Int. J. Pharm. 2020, 586, 119538. [Google Scholar] [CrossRef]
- Sattar, M.; Lane, M.E. Oral Transmucosal Delivery of Naratriptan. Int. J. Pharm. 2016, 514, 263–269. [Google Scholar] [CrossRef]
- Itin, C.; Domb, A.J.; Hoffman, A. A Meta-Opinion: Cannabinoids Delivered to Oral Mucosa by a Spray for Systemic Absorption Are Rather Ingested into Gastro-Intestinal Tract: The Influences of Fed/Fasting States. Expert Opin. Drug Deliv. 2019, 16, 1031–1035. [Google Scholar] [CrossRef]
- Watkinson, R.M.; Guy, R.H.; Oliveira, G.; Hadgraft, J.; Lane, M.E. Optimisation of Cosolvent Concentration for Topical Drug Delivery III--Influence of Lipophilic Vehicles on Ibuprofen Permeation. Skin Pharmacol. Physiol. 2011, 24, 22–26. [Google Scholar] [CrossRef]
- Diaz Del Consuelo, I.; Pizzolato, G.-P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of Pig Esophageal Mucosa as a Permeability Barrier Model for Buccal Tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef]
- Soe, M.T.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. Modified Glutinous Rice Starch-Chitosan Composite Films for Buccal Delivery of Hydrophilic Drug. Carbohydr. Polym. 2020, 245, 116556. [Google Scholar] [CrossRef]
- Rai, V.; Tan, H.S.; Michniak-Kohn, B. Effect of Surfactants and PH on Naltrexone (NTX) Permeation across Buccal Mucosa. Int. J. Pharm. 2011, 411, 92–97. [Google Scholar] [CrossRef]
- Sohi, H.; Ahuja, A.; Ahmad, F.J.; Khar, R.K. Critical Evaluation of Permeation Enhancers for Oral Mucosal Drug Delivery. Drug Dev. Ind. Pharm. 2010, 36, 254–282. [Google Scholar] [CrossRef]
- Majid, H.; Puzik, A.; Maier, T.; Merk, R.; Bartel, A.; Mueller, H.-C.; Burckhardt, B.B. Formulation Development of Sublingual Cyclobenzaprine Tablets Empowered by Standardized and Physiologically Relevant Ex Vivo Permeation Studies. Pharmaceutics 2021, 13, 1409. [Google Scholar] [CrossRef]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®-Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Lane, M.E. Skin Penetration Enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy: In Manufacture, Formulation and Clinical Use, 6th ed.; Pharmaceutical Press: London, UK, 2016. [Google Scholar]
- Kim, C. Advanced Pharmaceutics: Physiochemical Principles; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Sarode, A.V.; Kumbharkhane, A.C. Dielectric Relaxation Study of Poly(Ethylene Glycols) Using TDR Technique. J. Mol. Liq. 2011, 164, 226–232. [Google Scholar] [CrossRef]
- Harada, S.-I.; Horisawa, E.; Kano, S.; Sugibayashi, K. Formulation Study of Topically Applied O/W Lotion Containing Vitamin D3 Derivative, Focusing on Skin Permeability of the Drug. Drug Dev. Ind. Pharm. 2011, 37, 917–925. [Google Scholar] [CrossRef]
- Liu, B.; Du, Q.; Yang, Y. The Phase Diagrams of Mixtures of EVAL and PEG in Relation to Membrane Formation. J. Membr. Sci. 2000, 180, 81–92. [Google Scholar] [CrossRef]
- Kalam, M.A.; Alshamsan, A.; Alkholief, M.; Alsarra, I.A.; Ali, R.; Haq, N.; Anwer, M.K.; Shakeel, F. Solubility Measurement and Various Solubility Parameters of Glipizide in Different Neat Solvents. ACS Omega 2020, 5, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.N.; Sinko, P.J.; Singh, Y. (Eds.) Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 6th ed.; 50th Anniversary Edition; Lippincott Williams & Wilkins: Baltimore, MD, UAS, 2011. [Google Scholar]
- Majid, H.; Bartel, A.; Burckhardt, B.B. Predictivity of Standardized and Controlled Permeation Studies: Ex Vivo—In Vitro—In Vivo Correlation for Sublingual Absorption of Propranolol. Eur. J. Pharm. Biopharm. 2021, 169, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Fabin, B. Altered Skin Permeation of a Highly Lipophilic Molecule: Tetrahydrocannabinol. Int. J. Pharm. 1988, 43, 17–22. [Google Scholar] [CrossRef]
- Veuillez, F.; Rieg, F.F.; Guy, R.H.; Deshusses, J.; Buri, P. Permeation of a Myristoylated Dipeptide across the Buccal Mucosa: Topological Distribution and Evaluation of Tissue Integrity. Int. J. Pharm. 2002, 231, 1–9. [Google Scholar] [CrossRef]
- Kraisit, P.; Yonemochi, E.; Furuishi, T.; Mahadlek, J.; Limmatvapirat, S. Chitosan Film Containing Antifungal Agent-Loaded SLNs for the Treatment of Candidiasis Using a Box-Behnken Design. Carbohydr. Polym. 2022, 283, 119178. [Google Scholar] [CrossRef]
- Sharkawy, A.; Silva, A.M.; Rodrigues, F.; Barreiro, F.; Rodrigues, A. Pickering Emulsions Stabilized with Chitosan/Collagen Peptides Nanoparticles as Green Topical Delivery Vehicles for Cannabidiol (CBD). Colloids Surf. Physicochem. Eng. Asp. 2021, 631, 127677. [Google Scholar] [CrossRef]
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human Skin Permeation of Delta8-Tetrahydrocannabinol, Cannabidiol and Cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297. [Google Scholar] [CrossRef]
- Junaid, M.S.A.; Tijani, A.O.; Puri, A.; Banga, A.K. In Vitro Percutaneous Absorption Studies of Cannabidiol Using Human Skin: Exploring the Effect of Drug Concentration, Chemical Enhancers, and Essential Oils. Int. J. Pharm. 2022, 616, 121540. [Google Scholar] [CrossRef]
- Rathbone, M.J.; Tucker, I.G. Mechanisms, Barriers and Pathways of Oral Mucosal Drug Permeation. Adv. Drug Deliv. Rev. 1993, 12, 41–60. [Google Scholar] [CrossRef]
- Itin, C.; Barasch, D.; Domb, A.J.; Hoffman, A. Prolonged Oral Transmucosal Delivery of Highly Lipophilic Drug Cannabidiol. Int. J. Pharm. 2020, 581, 119276. [Google Scholar] [CrossRef]
- Ghafourian, T.; Nokhodchi, A.; Kaialy, W. Surfactants as Penetration Enhancers for Dermal and Transdermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 207–230. [Google Scholar]
- Limpongsa, E.; Jaipakdee, N.; Pongjanyakul, T. Skin Deposition and Permeation of Finasteride in Vitro: Effects of Propylene Glycol, Ethanol and Sodium Lauryl Sulfate. Pharm. Dev. Technol. 2015, 20, 984–991. [Google Scholar] [CrossRef]
- Caon, T.; Pan, Y.; Simões, C.M.O.; Nicolazzo, J.A. Exploiting the Buccal Mucosa as an Alternative Route for the Delivery of Donepezil Hydrochloride. J. Pharm. Sci. 2014, 103, 1643–1651. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Kung, C.-P.; Iliopoulos, F.; Sil, B.C.; Hadgraft, J.; Lane, M.E. Dermal Delivery of Niacinamide—In Vivo Studies. Pharmaceutics 2021, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical Delivery of Anthramycin I. Influence of Neat Solvents. Eur. J. Pharm. Sci. 2017, 104, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In Vitro-in Vivo Correlation in Skin Permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Monjur Al Hossain, A.S.M.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical Delivery of Niacinamide: Influence of Neat Solvents. Int. J. Pharm. 2020, 579, 119137. [Google Scholar] [CrossRef]
- Vasyuchenko, E.P.; Orekhov, P.S.; Armeev, G.A.; Bozdaganyan, M.E. CPE-DB: An Open Database of Chemical Penetration Enhancers. Pharmaceutics 2021, 13, 66. [Google Scholar] [CrossRef]
- Söpper, U.; Hoffmann, A.; Daniels, R. Mucoadhesion and Mucopenetration of Cannabidiol (CBD)-Loaded Mesoporous Carrier Systems for Buccal Drug Delivery. Sci. Pharm. 2021, 89, 35. [Google Scholar] [CrossRef]
- Harrison, J.E.; Watkinson, A.C.; Green, D.M.; Hadgraft, J.; Brain, K. The Relative Effect of Azone and Transcutol on Permeant Diffusivity and Solubility in Human Stratum Corneum. Pharm. Res. 1996, 13, 542–546. [Google Scholar] [CrossRef]
- Puglia, C.; Bonina, F. Effect of Polyunsaturated Fatty Acids and Some Conventional Penetration Enhancers on Transdermal Delivery of Atenolol. Drug Deliv. 2008, 15, 107–112. [Google Scholar] [CrossRef]
- Kung, C.-P.; Sil, B.C.; Hadgraft, J.; Lane, M.E.; Patel, B.; McCulloch, R. Preparation, Characterization and Dermal Delivery of Methadone. Pharmaceutics 2019, 11, 509. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical Delivery of Anthramycin II. Influence of Binary and Ternary Solvent Systems. Eur. J. Pharm. Sci. 2018, 121, 59–64. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef]
- Paudel, K.S.; Hammell, D.C.; Agu, R.U.; Valiveti, S.; Stinchcomb, A.L. Cannabidiol Bioavailability after Nasal and Transdermal Application: Effect of Permeation Enhancers. Drug Dev. Ind. Pharm. 2010, 36, 1088–1097. [Google Scholar] [CrossRef]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The Role of Vehicle Interactions on Permeation of an Active through Model Membranes and Human Skin. Int. J. Cosmet. Sci. 2012, 34, 536–545. [Google Scholar] [CrossRef]
- Sattar, M.; Hadgraft, J.; Lane, M.E. Preparation, Characterization and Buccal Permeation of Naratriptan. Int. J. Pharm. 2015, 493, 146–151. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In Vivo Investigations on the Penetration of Various Oils and Their Influence on the Skin Barrier. Ski. Res. Technol. 2012, 18, 364–369. [Google Scholar] [CrossRef]
- Lee, C.K.; Uchida, T.; Noguchi, E.; Kim, N.S.; Goto, S. Skin Permeation Enhancement of Tegafur by Ethanol/Panasate 800 or Ethanol/Water Binary Vehicle and Combined Effect of Fatty Acids and Fatty Alcohols. J. Pharm. Sci. 1993, 82, 1155–1159. [Google Scholar] [CrossRef]
- Uchida, T.; Lee, C.K.; Sekiya, N.; Goto, S. Enhancement Effect of an Ethanol/Panasate 800 Binary Vehicle on Anti-Inflammatory Drug Permeation across Excised Hairless Mouse Skin. Biol. Pharm. Bull. 1993, 16, 168–171. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.-D.; Chai, Y.-P.; Zhang, H.; Peng, P.; Yang, X.-X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef]
- Padula, C.; Pescina, S.; Nicoli, S.; Santi, P. New Insights on the Mechanism of Fatty Acids as Buccal Permeation Enhancers. Pharmaceutics 2018, 10, 201. [Google Scholar] [CrossRef]
- Wang, M.Y.; Yang, Y.Y.; Heng, P.W.S. Skin Permeation of Physostigmine from Fatty Acids-Based Formulations: Evaluating the Choice of Solvent. Int. J. Pharm. 2005, 290, 25–36. [Google Scholar] [CrossRef]
- Hansen, S.E.; Marxen, E.; Janfelt, C.; Jacobsen, J. Buccal Delivery of Small Molecules—Impact of Levulinic Acid, Oleic Acid, Sodium Dodecyl Sulfate and Hypotonicity on Ex Vivo Permeability and Spatial Distribution in Mucosa. Eur. J. Pharm. Biopharm. 2018, 133, 250–257. [Google Scholar] [CrossRef]





| Liquid Vehicles | CBD Solubility (mg/mL) | ||
|---|---|---|---|
| Types | Hansen Solubility Parameters (MPa1/2) * | Dielectric Constants ** | |
| MCT | 18.8 | 3.9 | >1000 *** |
| DEGEE | 21.4 | 14.1 | 598.0 ± 12.2 |
| PG | 29.2 | 32.1 | 514.0 ± 54.7 |
| PEG 300 | 22.5 | 14.5 | >1000 *** |
| Vehicles | Vehicle Uptake Capacity (%) 1 | Jss (µg·cm−2 h−1) 2 | Tlag (h) 2 | Q8h (µg) 2 | Ps (×10−3 cm h−1) 2 | Dss (×10−3 cm2 h−1) 2 | K 2 |
|---|---|---|---|---|---|---|---|
| MCT | 12.2 ± 2.4 a | 0.89 ± 0.15 a | 2.17 ± 0.18 a | 10.78 ± 1.71 a | 0.036 ± 0.006 a | 0.307 ± 0.031 a | 0.006 ± 0.002 a |
| DEGEE | 7.8 ± 0.9 b | 163.01 ± 18.89 b | 2.09 ± 0.31 a | 2009.41 ± 230.92 b | 6.510 ± 0.754 b | 0.320 ± 0.043 a | 1.287 ± 0.162 b |
| PG | 30.9 ± 2.6 c | 179.81 ± 23.46 b | 1.72 ± 0.25 a | 2312.41 ± 380.39 b | 7.181 ± 0.937 b | 0.386 ± 0.066 a | 1.173 ± 0.151 b |
| PEG 300 | 3.0 ± 0.1 d | 17.66 ± 3.12 a | 2.15 ± 0.10 a | 218.65 ± 35.89 a | 0.705 ± 0.125 a | 0.312 ± 0.012 a | 0.143 ± 0.024 a |
| PG:DEGEE Ratio | Vehicle Uptake Capacity (%) 1 | Jss (µg·cm−2 h−1) 2 | Tlag (h) 2 | Q8h (µg) 2 | Ps (×10−3 cm h−1) 2 | Dss (×10−3 cm2 h−1) 2 | K 2 | ER |
|---|---|---|---|---|---|---|---|---|
| 0:100 | 7.8 ± 0.9 a | 163.01 ± 18.89 a | 2.09 ± 0.31 a | 2009.41 ± 230.92 a | 6.510 ± 0.754 a | 0.320 ± 0.043 a | 1.287 ± 0.162 a | 1.0 |
| 25:75 | 13.3 ± 1.3 b | 244.74 ± 29.33 b | 1.84 ± 0.10 a | 3090.17 ± 407.06 b | 9.766 ± 1.170 b | 0.360 ± 0.012 a | 1.705 ± 0.143 b | 1.5 |
| 50:50 | 12.1 ± 1.2 b | 263.39 ± 46.68 b | 1.95 ± 0.05 a | 3272.40 ± 587.80 b | 10.494 ± 1.860 b | 0.330 ± 0.004 a | 1.980 ± 0.377 b | 1.6 |
| 100:0 | 30.9 ± 2.6 c | 179.81 ± 23.46 b | 1.72 ± 0.25 a | 2312.41 ± 380.39 b | 7.181 ± 0.937 b | 0.386 ± 0.066 a | 1.173 ± 0.151 b | N/A |
| DEGEE:MCT Ratio | Vehicle Uptake Capacity (%) 1 | Jss (µg·cm−2 h−1) 2 | Tlag (h) 2 | Q8h (µg) 2 | Ps (×10−3 cm h−1) 2 | Dss (×10−3 cm2 h−1) 2 | K 2 | ER |
|---|---|---|---|---|---|---|---|---|
| 0:100 | 12.2 ± 2.4 a | 0.89 ± 0.15 a | 2.17 ± 0.18 a | 10.78 ± 1.71 a | 0.036 ± 0.006 a | 0.307 ± 0.031 a | 0.006 ± 0.002 a | 1.0 |
| 25:75 | 14.4 ± 1.1 a | 16.34 ± 2.40 b | 0.23 ± 0.05 b | 254.95 ± 35.26 b | 0.651 ± 0.095 b | 2.984 ± 0.835 b | 0.014 ± 0.004 b | 17.7 |
| 50:50 | 8.6 ± 0.9 b | 38.84 ± 3.63 c | 0.25 ± 0.05 b | 583.04 ± 118.71 c | 1.551 ± 0.145 c | 2.623 ± 0.425 b | 0.037 ± 0.004 c | 43.8 |
| 75:25 | 8.1 ± 0.8 b | 9.48 ± 1.59 d | 0.26 ± 0.07 b | 147.13 ± 25.01 b | 0.379 ± 0.064 d | 2.716 ± 0.728 b | 0.009 ± 0.002 a | 10.9 |
| 100:0 | 7.8 ± 0.9 | 163.01 ± 18.89 | 2.09 ± 0.31 | 2009.41 ± 230.92 | 6.510 ± 0.754 | 0.320 ± 0.043 | 1.287 ± 0.162 | N/A |
| Vehicles | Vehicle Uptake Capacity (%) 1 | Jss (µg·cm−2 h−1) 2 | Tlag (h) 2 | Q8h (µg) 2 | Ps (×10−3 cm h−1) 2 | Dss (×10−3 cm2 h−1) 2 | K 2 | ER |
|---|---|---|---|---|---|---|---|---|
| Without enhancer | 13.3 ± 1.3 a | 244.74 ± 29.33 a | 1.84 ± 0.10 a | 3090.17 ± 407.06 a | 9.766 ± 1.170 a | 0.360 ± 0.012 a | 1.705 ± 0.143 a | 1.0 |
| Laurocapram | 11.9 ± 1.1 a | 98.60 ± 12.41 b | 0.46 ± 0.17 b | 1498.15 ± 218.21 b | 3.925 ± 0.494 b | 1.627 ± 0.739 b | 0.168 ± 0.052 b | 0.4 |
| Oleic acid | 8.6 ± 1.2 b | 159.39 ± 15.59 c | 1.66 ± 0.17 a | 2057.66 ± 232.81 b | 6.376 ± 0.624 c | 0.408 ± 0.050 a | 0.997 ± 0.093 c | 0.7 |
| Nerolidol | 14.1 ± 0.9 a | 224.14 ± 21.87 a | 1.70 ± 0.15 a | 2905.16 ± 274.28 a | 8.923 ± 0.871 a | 0.384 ± 0.026 a | 1.457 ± 0.178 d | 0.9 |
| Vehicles | Vehicle Uptake Capacity (%) 1 | Jss (µg·cm−2 h−1) 2 | Tlag (h) 2 | Q8h (µg) 2 | Ps (×10−3 cm·h−1) 2 | Dss (×10−3 cm2 h−1) 2 | K 2 | ER |
|---|---|---|---|---|---|---|---|---|
| Without enhancer | 14.4 ± 1.1 a | 16.34 ± 2.40 a | 0.23 ± 0.05 a | 254.95 ± 35.26 a | 0.651 ± 0.095 a | 2.984 ± 0.835 a | 0.014 ± 0.004 a | 1.0 |
| Laurocapram | 15.1 ± 0.7 a | 5.51 ± 0.54 b | 0.54 ± 0.18 b | 81.13 ± 6.82 b | 0.220 ± 0.022 b | 1.332 ± 0.562 b | 0.012 ± 0.002 a | 0.3 |
| Oleic acid | 13.6 ± 0.8 a | 6.71 ± 0.45 b | 0.19 ± 0.04 a | 103.60 ± 10.11 b | 0.268 ± 0.018 b | 3.727 ± 0.905 a | 0.005 ± 0.001 b | 0.4 |
| Nerolidol | 11.0 ± 1.2 b | 19.29 ± 1.95 a | 0.19 ± 0.05 a | 297.53 ± 30.64 a | 0.770 ± 0.078 a | 3.610 ± 0.589 a | 0.014 ± 0.003 a | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabboon, P.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. Mucosal Delivery of Cannabidiol: Influence of Vehicles and Enhancers. Pharmaceutics 2022, 14, 1687. https://doi.org/10.3390/pharmaceutics14081687
Tabboon P, Pongjanyakul T, Limpongsa E, Jaipakdee N. Mucosal Delivery of Cannabidiol: Influence of Vehicles and Enhancers. Pharmaceutics. 2022; 14(8):1687. https://doi.org/10.3390/pharmaceutics14081687
Chicago/Turabian StyleTabboon, Peera, Thaned Pongjanyakul, Ekapol Limpongsa, and Napaphak Jaipakdee. 2022. "Mucosal Delivery of Cannabidiol: Influence of Vehicles and Enhancers" Pharmaceutics 14, no. 8: 1687. https://doi.org/10.3390/pharmaceutics14081687