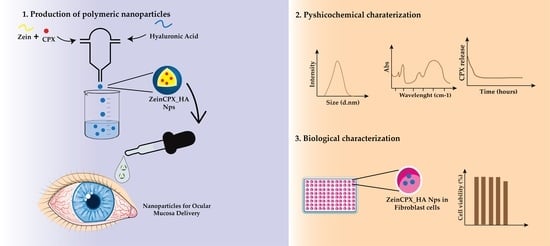
Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of the Zein and HA-Based NPs
2.3. Screening of Cryoprotectants
2.4. Characterization of the NPs
2.4.1. Particle Dh, Zeta Potential and Polydispersity Index (PDI) Measurements
2.4.2. Fourier Transform Infrared (FTIR) Analysis
2.4.3. Thermogravimetric (TGA) and Differential Scanning Calorimetry (DSC) Analysis
2.5. Production of ZeinCPX_HA NPs and Stability Studies
2.6. Encapsulation Efficiency and Loading Capacity
2.7. In Vitro Drug Release of CPX-Loaded NPs
2.8. Biocompatibility Assay
3. Results and Discussion
3.1. Effect of Polymers’ Concentration on Their Properties
3.2. Selection of the Cryoprotectant
3.3. Characterization of NPs Incorporating CPX
3.4. Encapsulation Efficiency and Drug Loading
3.5. CPX Release Profile from ZeinCPX_HA NPs
3.6. Characterization of NPs’ Biological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haq, A.; Wardak, H.; Kraski, N. Infective Conjunctivitis—Its Pathogenesis, Management and Complications. In Common Eye Infections; Chaudhry, I.A., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Azari, A.A.; Arabi, A. Conjunctivitis: A Systematic Review. J. Ophthalmic Vis. Res. 2020, 15, 372–395. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Cabrera-Aguas, M.; Khoo, P. Common eye infections. Aust. Prescr. 2018, 41, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Benitez-del-Castillo, J.; Verboven, Y.; Stroman, D.; Kodjikian, L. The Role of Topical Moxifloxacin, a New Antibacterial in Europe, in the Treatment of Bacterial Conjunctivitis. Clin. Drug Investig. 2011, 31, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Pippin, M.M.; Le, J.K. Bacterial Conjunctivitis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546683/ (accessed on 18 June 2022).
- Alharbi, W.S.; Hosny, K.M. Development and optimization of ocular in situ gels loaded with ciprofloxacin cubic liquid crystalline nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101710. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The new fluoroquinolones: A critical review. Can. J. Infect. Dis. 1999, 10, 207–238. [Google Scholar] [CrossRef]
- Taha, E.I.; El-Anazi, M.H.; El-Bagory, I.M.; Bayomi, M.A. Design of liposomal colloidal systems for ocular delivery of ciprofloxacin. Saudi Pharm. J. 2014, 22, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Al-Joufi, F.A.; Salem-Bekhit, M.M.; Taha, E.I.; Ibrahim, M.A.; Muharram, M.M.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Enhancing Ocular Bioavailability of Ciprofloxacin Using Colloidal Lipid-Based Carrier for the Management of Post-Surgical Infection. Molecules 2022, 27, 733. [Google Scholar] [CrossRef]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef]
- Meza-Rios, A.; Navarro-Partida, J.; Armendariz-Borunda, J.; Santos, A. Therapies Based on Nanoparticles for Eye Drug Delivery. Ophthalmol. Ther. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Omerović, N.; Vranić, E. Application of nanoparticles in ocular drug delivery systems. Health Technol. 2020, 10, 61–78. [Google Scholar] [CrossRef]
- Vaneev, A.; Tikhomirova, V.; Chesnokova, N.; Popova, E.; Beznos, O.; Kost, O.; Klyachko, N. Nanotechnology for Topical Drug Delivery to the Anterior Segment of the Eye. Int. J. Mol. Sci. 2021, 22, 12368. [Google Scholar] [CrossRef]
- Ban, J.; Zhang, Y.; Huang, X.; Deng, G.; Hou, D.; Chen, Y.; Lu, Z. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int. J. Nanomed. 2017, 12, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Massella, D.; Celasco, E.; Salaün, F.; Ferri, A.; Barresi, A.A. Overcoming the Limits of Flash Nanoprecipitation: Effective Loading of Hydrophilic Drug into Polymeric Nanoparticles with Controlled Structure. Polymers 2018, 10, 1092. [Google Scholar] [CrossRef] [Green Version]
- Pustulka, K.M.; Wohl, A.R.; Lee, H.S.; Michel, A.R.; Han, J.; Hoye, T.R.; McCormick, A.V.; Panyam, J.; Macosko, C.W. Flash Nanoprecipitation: Particle Structure and Stability. Mol. Pharm. 2013, 10, 4367–4377. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, J.; Miguel, S.P.; Seabra, I.J.; Ribeiro, M.P.; Coutinho, P. Single-Step Self-Assembly of Zein–Honey–Chitosan Nanoparticles for Hydrophilic Drug Incorporation by Flash Nanoprecipitation. Pharmaceutics 2022, 14, 920. [Google Scholar] [CrossRef]
- Miguel, S.P.; Loureiro, J.; Ribeiro, M.P.; Coutinho, P. Osmundea sp. macroalgal polysaccharide-based nanoparticles produced by flash nanocomplexation technique. Int. J. Biol. Macromol. 2022, 204, 9–18. [Google Scholar] [CrossRef]
- Nunes, R.; Baião, A.; Monteiro, D.; das Neves, J.; Sarmento, B. Zein nanoparticles as low-cost, safe, and effective carriers to improve the oral bioavailability of resveratrol. Drug Deliv. Transl. Res. 2020, 10, 826–837. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Wu, H.; Hu, H.; Dong, Z.; Dang, Y.; Qi, Q.; Wang, Y.; Du, S.; Lu, Y. Zein nanoparticles as nontoxic delivery system for maytansine in the treatment of non-small cell lung cancer. Drug Deliv. 2020, 27, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Padua, G.W.; Wang, Y. Controlled formation of hydrophobic surfaces by self-assembly of an amphiphilic natural protein from aqueous solutions. Soft Matter 2013, 9, 5933–5941. [Google Scholar] [CrossRef]
- Rodrigues, D.A.; Miguel, S.P.; Loureiro, J.; Ribeiro, M.; Roque, F.; Coutinho, P. Oromucosal Alginate Films with Zein Nanoparticles as a Novel Delivery System for Digoxin. Pharmaceutics 2021, 13, 2030. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Salvatici, M.C.; Fresta, M.; Cosco, D. Brij-stabilized zein nanoparticles as potential drug carriers. Colloids Surf. B Biointerfaces 2021, 201, 111647. [Google Scholar] [CrossRef]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in Ocular Drug Delivery. Pharmaceutics 2020, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic acid in ocular drug delivery. Carbohydr. Polym. 2021, 264, 118006. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Jung, M.-Y.; Pak, H.-J.; Park, J.-H.; Kim, M.; Chuck, R.S.; Park, C.Y. Development of a novel hyaluronic acid membrane for the treatment of ocular surface diseases. Sci. Rep. 2021, 11, 2351. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Papa, V.; Micali, A.; Santocono, M.; Milazzo, G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br. J. Ophthalmol. 2002, 86, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, J.; Hall, C.L.; Turley, E.A. HA receptors: Regulators of signalling to the cytoskeleton. J. Cell. Biochem. 1996, 61, 569–577. [Google Scholar] [CrossRef]
- Kalam, M.A. The potential application of hyaluronic acid coated chitosan nanoparticles in ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 559–568. [Google Scholar] [CrossRef]
- Butreddy, A.; Dudhipala, N.; Janga, K.Y.; Gaddam, R.P. Lyophilization of Small-Molecule Injectables: An Industry Perspective on Formulation Development, Process Optimization, Scale-Up Challenges, and Drug Product Quality Attributes. AAPS PharmSciTech 2020, 21, 252. [Google Scholar] [CrossRef]
- Rayaprolu, B.M.; Strawser, J.J.; Anyarambhatla, G. Excipients in parenteral formulations: Selection considerations and effective utilization with small molecules and biologics. Drug Dev. Ind. Pharm. 2018, 44, 1565–1571. [Google Scholar] [CrossRef]
- Han, J.; Zhu, Z.; Qian, H.; Wohl, A.R.; Beaman, C.J.; Hoye, T.R.; Macosko, C.W. A simple confined impingement jets mixer for flash nanoprecipitation. J. Pharm. Sci. 2012, 101, 4018–4023. [Google Scholar] [CrossRef]
- Hong, D.Y.; Lee, J.-S.; Lee, H.G. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int. J. Biol. Macromol. 2016, 85, 9–15. [Google Scholar] [CrossRef]
- Voci, S.; Gagliardi, A.; Salvatici, M.C.; Fresta, M.; Cosco, D. Influence of the Dispersion Medium and Cryoprotectants on the Physico-Chemical Features of Gliadin- and Zein-Based Nanoparticles. Pharmaceutics 2022, 14, 332. [Google Scholar] [CrossRef]
- Ministério da Saúde, I. Farmacopeia Portuguesa 9; Instituto Nacional da Farmacia e do Medicamento: Ponta Delgada, Portugal, 2008. [Google Scholar]
- Liu, Y.; Liu, J.; Zhang, X.; Zhang, R.; Huang, Y.; Wu, C. In Situ Gelling Gelrite/Alginate Formulations as Vehicles for Ophthalmic Drug Delivery. AAPS PharmSciTech 2010, 11, 610–620. [Google Scholar] [CrossRef] [Green Version]
- Paulsson, M.; Hägerström, H.; Edsman, K. Rheological studies of the gelation of deacetylated gellan gum (Gelrite®) in physiological conditions. Eur. J. Pharm. Sci. 1999, 9, 99–105. [Google Scholar] [CrossRef]
- Dave, R.S.; Goostrey, T.C.; Ziolkowska, M.; Czerny-Holownia, S.; Hoare, T.; Sheardown, H. Ocular drug delivery to the anterior segment using nanocarriers: A mucoadhesive/mucopenetrative perspective. J. Control Release 2021, 336, 71–88. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Gu, Y.; Liu, Y.; Zhao, J.; Yan, B.; Wang, Y. Cryoprotectant choice and analyses of freeze-drying drug suspension of nanoparticles with functional stabilisers. J. Microencapsul. 2018, 35, 241–248. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y.; McManus, S.A.; Qian, R.; Ristroph, K.D.; Ramachandruni, H.; Gong, K.; White, C.E.; Rawal, A.; Prud’homme, R.K. Amorphous nanoparticles by self-assembly: Processing for controlled release of hydrophobic molecules. Soft Matter 2019, 15, 2400–2410. [Google Scholar] [CrossRef]
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone-hyaluronic acid/chitosan-zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, G.; Liu, X.; Ren, Q.; Huang, F.; Yan, Y. Fabrication of polysaccharide-stabilized zein nanoparticles by flash nanoprecipitation for doxorubicin sustained release. J. Drug Deliv. Sci. Technol. 2022, 70, 103183. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Liu, C.; Zhu, J.; Xu, Y.; Zhang, S.; Fan, M.; Zhang, D.; Zhang, Y.; Zhang, Z.; et al. Fabrication of stable zein nanoparticles by chondroitin sulfate deposition based on antisolvent precipitation method. Int. J. Biol. Macromol. 2019, 139, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, T.A.; Rodrigues, C.F.; Moreira, A.F.; Miguel, S.P.; Costa, E.C.; Ferreira, P.; Correia, I.J. Hyaluronic acid and vitamin E polyethylene glycol succinate functionalized gold-core silica shell nanorods for cancer targeted photothermal therapy. Colloids Surf. B Biointerfaces 2020, 188, 110778. [Google Scholar] [CrossRef]
- Sakulwech, S.; Lourith, N.; Ruktanonchai, U.; Kanlayaavattanakul, M. Preparation and characterization of nanoparticles from quaternized cyclodextrin-grafted chitosan associated with hyaluronic acid for cosmetics. Asian J. Pharm. Sci. 2018, 13, 498–504. [Google Scholar] [CrossRef]
- Demirci, S.; Celebioglu, A.; Aytac, Z.; Uyar, T. pH-responsive nanofibers with controlled drug release properties. Polym. Chem. 2014, 5, 2050–2056. [Google Scholar] [CrossRef] [Green Version]
- Devanand Venkatasubbu, G.; Ramasamy, S.; Ramakrishnan, V.; Kumar, J. Nanocrystalline hydroxyapatite and zinc-doped hydroxyapatite as carrier material for controlled delivery of ciprofloxacin. 3 Biotech 2011, 1, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Dong, Z.; Du, Y.; Kennedy, J.F. Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohydr. Polym. 2007, 69, 336–343. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Rosqvist, E.; Smått, J.-H.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and optimization of lyophilized nanosuspension tablets to improve the physicochemical properties and provide immediate release of silymarin. Int. J. Pharm. 2019, 563, 217–227. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef]
- Ahire, J.J.; Robertson, D.; Neveling, D.P.; van Reenen, A.J.; Dicks, L.M.T. Hyaluronic acid-coated poly(d,l-lactide) (PDLLA) nanofibers prepared by electrospinning and coating. RSC Adv. 2016, 6, 34791–34796. [Google Scholar] [CrossRef] [Green Version]
- Dhayanandamoorthy, Y.; Antoniraj, M.G.; Kandregula, C.A.B.; Kandasamy, R. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: A promising asthma control strategy. Int. J. Pharm. 2020, 591, 119958. [Google Scholar] [CrossRef]
- Acevedo, F.; Hermosilla, J.; Sanhueza, C.; Mora-Lagos, B.; Fuentes, I.; Rubilar, M.; Concheiro, A.; Alvarez-Lorenzo, C. Gallic acid loaded PEO-core/zein-shell nanofibers for chemopreventive action on gallbladder cancer cells. Eur. J. Pharm. Sci. 2018, 119, 49–61. [Google Scholar] [CrossRef]
- Abate, M.; Scotti, L.; Nele, V.; Caraglia, M.; Biondi, M.; De Rosa, G.; Leonetti, C.; Campani, V.; Zappavigna, S.; Porru, M. Hybrid Self-Assembling Nanoparticles Encapsulating Zoledronic Acid: A Strategy for Fostering Their Clinical Use. Int. J. Mol. Sci. 2022, 23, 5138. [Google Scholar]
- Liu, J.; Grohganz, H.; Löbmann, K.; Rades, T.; Hempel, N.-J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics 2021, 13, 389. [Google Scholar]
- Stewart, A.M.; Grass, M.E. Practical Approach to Modeling the Impact of Amorphous Drug Nanoparticles on the Oral Absorption of Poorly Soluble Drugs. Mol. Pharm. 2020, 17, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Mohamed, H.B.; Attia Shafie, M.A.; Mekkawy, A.I. Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model. Pharmaceutics 2022, 14, 893. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Castro-López, V.; Pañeda, C.; Alonso, M.J. Synthetic nanocarriers for the delivery of polynucleotides to the eye. Eur. J. Pharm. Sci. 2017, 103, 5–18. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Busch, M.; Asin-Prieto, E.; Peynshaert, K.; Rathod, R.; Remaut, K.; Dünker, N.; Göpferich, A. Hyaluronic acid coating of gold nanoparticles for intraocular drug delivery: Evaluation of the surface properties and effect on their distribution. Exp. Eye Res. 2020, 198, 108151. [Google Scholar] [CrossRef]
- Jin, Y.; Ubonvan, T.; Kim, D.-D. Hyaluronic Acid in Drug Delivery Systems. J. Pharm. Investig. 2010, 40, 33–43. [Google Scholar]
- Widjaja, L.K.; Bora, M.; Chan, P.N.P.H.; Lipik, V.; Wong, T.T.L.; Venkatraman, S.S. Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications. J. Biomed. Mater. Res. Part A 2014, 102, 3056–3065. [Google Scholar] [CrossRef]
- Bongiovì, F.; Di Prima, G.; Palumbo, F.S.; Licciardi, M.; Pitarresi, G.; Giammona, G. Hyaluronic Acid-Based Micelles as Ocular Platform to Modulate the Loading, Release, and Corneal Permeation of Corticosteroids. Macromol. Biosci. 2017, 17, 1700261. [Google Scholar] [CrossRef]
- Ricci, F.; Racaniello, G.F.; Lopedota, A.; Laquintana, V.; Arduino, I.; Lopalco, A.; Cutrignelli, A.; Franco, M.; Sigurdsson, H.H.; Denora, N. Chitosan/sulfobutylether-β-cyclodextrin based nanoparticles coated with thiolated hyaluronic acid for indomethacin ophthalmic delivery. Int. J. Pharm. 2022, 622, 121905. [Google Scholar] [CrossRef]
- Zeng, W.; Li, Q.; Wan, T.; Liu, C.; Pan, W.; Wu, Z.; Zhang, G.; Pan, J.; Qin, M.; Lin, Y.; et al. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: Mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Colloids Surf. B Biointerfaces 2016, 141, 28–35. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; El-Telbany, D.F.A.; Zayed, G.; Al-Sawahli, M.M. Hydrogel Containing PEG-Coated Fluconazole Nanoparticles with Enhanced Solubility and Antifungal Activity. J. Pharm. Innov. 2019, 14, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Joseph, E.; Singhvi, G. Chapter 4—Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–116. [Google Scholar]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef] [PubMed]
- Leigue, L.; Montiani-Ferreira, F.; Moore, B.A. Antimicrobial susceptibility and minimal inhibitory concentration of Pseudomonas aeruginosa isolated from septic ocular surface disease in different animal species. Open Vet. J. 2016, 6, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundada, A.S.; Shrikhande, B.K. Formulation and evaluation of ciprofloxacin hydrochloride soluble ocular drug insert. Curr. Eye Res. 2008, 33, 469–475. [Google Scholar] [CrossRef]
- Günday, C.; Anand, S.; Gencer, H.B.; Munafò, S.; Moroni, L.; Fusco, A.; Donnarumma, G.; Ricci, C.; Hatir, P.C.; Türeli, N.G.; et al. Ciprofloxacin-loaded polymeric nanoparticles incorporated electrospun fibers for drug delivery in tissue engineering applications. Drug Deliv. Transl. Res. 2020, 10, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-X.; Wang, H.-J.; Zhou, Y.-Q.; Wang, J.-Y. Antibacterial activity of ciprofloxacin-loaded zein microsphere films. Mater. Sci. Eng. C 2009, 29, 1161–1166. [Google Scholar] [CrossRef]
- Arafa, M.G.; Mousa, H.A.; Afifi, N.N. Preparation of PLGA-chitosan based nanocarriers for enhancing antibacterial effect of ciprofloxacin in root canal infection. Drug Deliv. 2020, 27, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.K.; Tripathi, M.; Pandey, N.; Agrawal, A.K.; Gade, S.; Anjum, M.M.; Tilak, R.; Singh, S. Alginate lyase immobilized chitosan nanoparticles of ciprofloxacin for the improved antimicrobial activity against the biofilm associated mucoid P. aeruginosa infection in cystic fibrosis. Int. J. Pharm. 2019, 563, 30–42. [Google Scholar] [CrossRef]
- Gheffar, C.; Le, H.; Jouenne, T.; Schaumann, A.; Corbière, A.; Vaudry, D.; LeCerf, D.; Karakasyan, C. Antibacterial Activity of Ciprofloxacin-Loaded Poly(lactic-co-glycolic acid)-Nanoparticles Against Staphylococcus aureus. Part. Part. Syst. Charact. 2021, 38, 2000253. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Jiang, X.; Gui, Z.; Zhang, L. Development of Hydrophilic Drug Encapsulation and Controlled Release Using a Modified Nanoprecipitation Method. Processes 2019, 7, 331. [Google Scholar] [CrossRef] [Green Version]
- Brotons-Canto, A.; González-Navarro, C.J.; Gil, A.G.; Asin-Prieto, E.; Saiz, M.J.; Llabrés, J.M. Zein Nanoparticles Improve the Oral Bioavailability of Curcumin in Wistar Rats. Pharmaceutics 2021, 13, 361. [Google Scholar] [CrossRef]
- Ghalei, S.; Asadi, H.; Ghalei, B. Zein nanoparticle-embedded electrospun PVA nanofibers as wound dressing for topical delivery of anti-inflammatory diclofenac. J. Appl. Polym. Sci. 2018, 135, 46643. [Google Scholar] [CrossRef]
- Lin, M.; Fang, S.; Zhao, X.; Liang, X.; Wu, D. Natamycin-loaded zein nanoparticles stabilized by carboxymethyl chitosan: Evaluation of colloidal/chemical performance and application in postharvest treatments. Food Hydrocoll. 2020, 106, 105871. [Google Scholar] [CrossRef]
- Reboredo, C.; González-Navarro, C.J.; Martínez-López, A.L.; Martínez-Ohárriz, C.; Sarmento, B.; Irache, J.M. Zein-Based Nanoparticles as Oral Carriers for Insulin Delivery. Pharmaceutics 2022, 14, 39. [Google Scholar] [CrossRef]
- Saraswathy, K.; Agarwal, G.; Srivastava, A. Hyaluronic acid microneedles-laden collagen cryogel plugs for ocular drug delivery. J. Appl. Polym. Sci. 2020, 137, 49285. [Google Scholar] [CrossRef]
- Sabra, S.A.; Elzoghby, A.O.; Sheweita, S.A.; Haroun, M.; Helmy, M.W.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Rohani, S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur. J. Pharm. Biopharm. 2018, 128, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Labib, B.A.; Chigbu, D.I. Therapeutic Targets in Allergic Conjunctivitis. Pharmaceuticals 2022, 15, 547. [Google Scholar] [CrossRef]
- Fukuda, K. Corneal fibroblasts: Function and markers. Exp. Eye Res. 2020, 200, 108229. [Google Scholar] [CrossRef]







| Polymers | Dh (nm) | PDI |
|---|---|---|
| HA (2.5 mg/mL) + Zein (2.5 mg/mL) | 146.1 ± 7.4 | 0.523 ± 0.019 |
| HA (2.5 mg/mL) + Zein (5 mg/mL) | 230.3 ± 15.1 | 0.853 ± 0.056 |
| HA (1 mg/mL) + Zein (2.5 mg/mL) | 86 ± 14 | 0.21 ± 0.07 |
| Cryoprotectant | RDI (%) |
|---|---|
| Mannitol 5% | 116 ± 32 |
| Mannitol 10% | 187 ± 72 |
| Mathematical Model | R2 | n |
|---|---|---|
| Zero order | 0.7657 | - |
| First order | 0.5791 | - |
| Higuchi | 0.9269 | - |
| Korsmeye–Peppas | 0.9505 | 0.2635 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacinto, T.A.; Oliveira, B.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery. Pharmaceutics 2022, 14, 1557. https://doi.org/10.3390/pharmaceutics14081557
Jacinto TA, Oliveira B, Miguel SP, Ribeiro MP, Coutinho P. Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery. Pharmaceutics. 2022; 14(8):1557. https://doi.org/10.3390/pharmaceutics14081557
Chicago/Turabian StyleJacinto, Telma A., Breno Oliveira, Sónia P. Miguel, Maximiano P. Ribeiro, and Paula Coutinho. 2022. "Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery" Pharmaceutics 14, no. 8: 1557. https://doi.org/10.3390/pharmaceutics14081557