1. Introduction
Glaucoma is a multifactorial long term ocular neuropathy, which is associated with a progressive loss of visual field, structural abnormalities of retinal nerve fiber and cupping of the optic nerve head [
1,
2]. Recently, it has become the second leading cause of blindness worldwide after cataracts [
3]. It was estimated that the primary open angle glaucoma cases in adult population will be risen up to 79.76 million in 2040 [
4]. Many predictors for glaucoma have been identified, including age, positive family history, race, myopia and exfoliation syndrome [
5]. Currently, intraocular pressure (IOP) is a major known risk factor for glaucoma. To lower IOP, treatment options involve oral and topical medications, laser therapy and surgical operation. Effective drug therapies include the drugs that reduce the rate of aqueous humor production and/or enhance its drainage. Several classes of drugs are available in managing long-term treatment of glaucoma, such as prostaglandin analogues, carbonic anhydrase inhibitors, α-adrenergic agonists, β-adrenergic blockers, and cholinergic agonists [
1,
2].
Angiotensin-converting enzyme (ACE) inhibitors have recently received attention as a new class of drug possessing the ability to lower IOP to treat glaucoma [
6,
7,
8]. ACE is responsible for the conversion of the biologically inactive angiotensin I to the potent vasopressor, angiotensin II as well as the breakdown of bradykinin. Inhibition of ACE leads to the accumulation of bradykinin and promote the synthesis of prostaglandins, which could in turn lower IOP by increasing the uveoscleral outflow [
9]. They also have a beneficial effect on retarding the progression of diabetic retinopathy in type II diabetic patients [
10,
11]. Moreover, ACE inhibitors showed beneficial effect in age-related macular degeneration [
12]. Of these, fosinopril (FOS), the ester prodrug of fosinoprilat, and the first orally active phosphorus-containing ACE inhibitor, is an interesting compound to be used for lowering IOP. However, hydrolysis degradation of FOS was found in all conditions, i.e., acidic, basic and neutral, whereas the greater extent in basic condition [
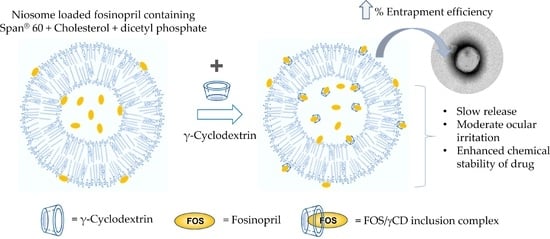
13]. Our previous study reported that the application of γ-cyclodextrin (γCD) as an inclusion complex could be able to enhance the solubility and chemical stability of FOS in aqueous solution [
14].
Recently, colloidal drug delivery has been introduced as an alternative formulation approach for problematic drug candidates. Numerous colloidal carriers such as liposomes, niosomes, nanoparticles, microemulsions and micelles have been developed, which are applicable not only to solving the problems of poor solubility and stability but also to providing specific drug targeting, optimizing drug release properties and reducing toxicity [
15]. As a vesicular carrier, niosome has gained attention because of its advantages including: (i) enhanced solubility and permeability; (ii) improved chemical stability; (iii) simple and cost-effective fabrication and (iv) low toxicity and high compatibility because of their nonionic nature [
16].
Niosomes are nonionic surfactant vesicles, rising from the self-assembly of nonionic amphiphiles in aqueous media. The spherical shaped niosomes are capable of entrapping lipophilic molecules within the lipid bilayer by interacting with alkyl chains of nonionic surfactants, whereas hydrophilic drug molecules are located within an aqueous core by interacting with polar head groups of nonionic surfactants [
17,
18]. Numerous studies have reported the successful use of niosomes as ocular drug delivery carriers [
19,
20,
21,
22,
23]. Vesicular delivery systems used in ophthalmic applications offer targeting at the site of action, improving chemical stability of encapsulated drugs and providing controlled release action at the corneal surface [
24,
25]. Vyas et al. (1998) reported that the ocular bioavailability of niosome entrapped water-soluble drugs, i.e., timolol maleate, increased as compared with timolol maleate solution [
19]. This can be explained in that surfactants behave as penetration enhancers by removing the mucus layer and breaking junctional complexes [
26].
In this study, niosomal eye drop preparations containing FOS alone or FOS/γCD inclusion complex were developed. The combined strategies, i.e., CD inclusion complex incorporated into a niosomal vesicle was applied to increase the chemical stability and to provide controlled drug release action. The physicochemical and chemical properties of niosomal formulations were evaluated. In addition, in vitro release, ex vivo permeation, in vitro cytotoxicity, and physical and chemical stability studies were also determined.
2. Materials and Methods
2.1. Materials
Fosinopril sodium (FOS) was purchased from Dideu Industries Group, Ltd. (Shaanxi, China). γ-Cyclodextrin (γCD) was purchased from Cyclolab (Budapest, Hungary). Polyoxyethylene 10 stearyl ether (Brij® 76) was distributed by The East Asiatic Public Company Ltd., (Bangkok, Thailand). Sorbitan monostearate (Span® 60) and poly-24-oxyethylene cholesteryl ether (Solulan® C-24, SC24) were kindly donated by Chemico Inter Corporation Ltd. (Bangkok, Thailand). Cholesterol, dicetyl phosphate (DCP) and stearylamine (STA) were received from Sigma-Aldrich (St. Louis, MO, USA), ethylenediamine tetra-acetic acid disodium salt (EDTA) and sodium metabisulfite (Na-MS) from Ajax Finechem Pty Ltd. (Taren Point, Australia). Semi-permeable cellophane membranes (SpectaPor®, molecular weight cut-off (MWCO) 12–14,000 Da) were obtained from Spectrum Europe (Breda, The Netherlands). All other chemicals used were of analytical reagent grade purity. Milli-Q (Millipore, Billerica, MA, USA) water was used to prepare all solutions.
2.2. Preparation of Niosomal Formulations Containing FOS
Niosome was prepared using thin-film hydration method. The niosome formulations were composed of nonionic surfactant, cholesterol, and membrane stabilizer/charged inducer at the mole ratio of 47.5: 47.5: 5. This ratio was optimized and shown to possess relatively good physicochemical characteristics obtained from blank niosome preparations. The total lipid composition was prepared at 100 μM in 5 mL of hydration medium (10 mM phosphate-buffered saline (pH 7.4) containing 1% (
w/v) FOS, 0.1% (
w/v) EDTA and 0.1% (
w/v) Na-MS). The surfactants used in this study included Span
® 60 and Brij
® 76. Nonionic SC24 was used as a steric stabilizer, while positively charged STA and negatively charged DCP were used to provide the electrostatic stabilization of vesicles. Briefly, accurately weighed amounts of nonionic surfactant, cholesterol and membrane stabilizer/charge inducer were dissolved in 10 mL of chloroform in a 1 L round-bottom flask. The lipid mixture was slowly evaporated under reduced pressure at 40 °C using a rotary evaporator (Rotavapor R-200, BÜCHI Labortechnik AG, Flawil, Switzerland) with a constant rotation speed. The flask was partially immersed in a water bath and evaporated until a dried thin film appeared on the inner wall of the flask. Then, the formulation was kept in a desiccator under vacuum for 2 h to ensure the total removal of trace solvents. After that, dried lipid film was hydrated with 5 mL of hydration medium with and without 5% (
w/v) γCD. Our previous work reported that EDTA and Na-MS are powerful antioxidants to protect FOS degradation [
14]. The hydration of dried film was carried out by rotating the flask in a water bath at 60 °C for 30 min using a rotavapor under normal pressure. The size reduction was made by sonicating in an ultrasonic bath (GT sonic, GT SONIC Technology Park, Guangdong, China) at 60 °C for 30 min. To complete annealing and partition of the drug between the lipid bilayer and the aqueous phase, the formulation was left overnight at room temperature and then stored at 4 °C until subjected to analysis. The compositions of niosome formulae are shown in
Table 1.
2.3. Physicochemical and Chemical Characterizations
2.3.1. Osmolality, pH and Viscosity Determination
The pH values of all formulations were measured using a pH meter (SevenCompact S220-Micro, Mettler Toledo, Gießen, Germany) at 25 °C. The viscosity was determined by viscometer (Sine-wave Vibro SV-10, A&D Company, Limited, Tokyo, Japan) using the tuning-fork vibration method with frequency of 30 Hz at 25 °C and 34 °C. The osmolality was determined by osmometer (OSMOMAT 3000 basic, Gonotec GmbH, Berlin, Germany) at room temperature using the freezing point depression principle. All measurements were determined in triplicate.
2.3.2. Particle Size, Size Distribution, and Zeta Potential
The particle size, size distribution and zeta potential of FOS-loaded niosome formulations were measured using the dynamic light scattering (DLS) technique (Zetasizer TM Nano ZS with software, Version 7.11, Malvern, UK). The measurements were carried out at a scattering angle of 180° and a temperature of 25 °C, a medium viscosity of 0.8872 mPa.s and a medium refractive index of 1.330. The concentration of niosome preparation was 20 μM. The particle size distribution was expressed as polydispersity (PDI). The particle size, size distribution and zeta potential were automatically calculated and analyzed using the software included within the system. Each measurement was performed in triplicate.
2.3.3. Determining Drug Content and Entrapment Efficiency (EE)
The FOS was quantitatively determined using a reversed-phase HPLC component system from Agilent 1260 Infinity II consisting of a liquid chromatography pump (quaternary pump, G7111A), diode array UV-Vis detector (DAD, G7115A), auto sampler (G7129A) with Chem Station Software, Version E.02.02 and Phenomenex Kinetex 5 µm C18 reverse-phase column (150 × 4.6 mm) with C18 guard cartridge column MG II 5 μm, 4 × 10 mm. The HPLC conditions were as described below. The mobile phase comprised aqueous solution containing 1% (v/v) tetrahydrofuran and 0.05% (v/v) phosphoric acid: acetonitrile (30:70 volume ratio); a flow rate of 0.9 mL/min; wavelength of 205 nm; injection volume of 20 μL; column oven temperature of 40 °C; and run time of 6 min. The analytical method validation was performed to satisfy the validation criteria.
Total FOS content in niosomal preparation was determined by dissolving 100 µL of the sample in 10 mL of methanol:water (50:50
v/v). After proper dilution, the solution was filtered through a 0.45 μm nylon filter and analyzed using HPLC. To determine the percentage of EE (%EE), the sample was ultra-centrifuged (CP100NX, Hitachi Koki Co., Ltd., Tokyo, Japan) at 18,000 rpm at 4 °C for 1 h. Then, the content of unentrapped drug in the supernatant was diluted with methanol: water (50:50
v/v) and quantified by HPLC. All samples were performed in triplicate. The %EE was calculated as Equation (1):
where
Dt is the total FOS content and
Ds is the FOS content in the supernatant.
2.3.4. Transmission Electron Microscopy (TEM) Analysis
The morphologic examinations of selected FOS-loaded niosomes with or without γCD were performed using the TEM technique. Initially, the sample was placed on a formvar-coated grid. After blotting the grid with a filter paper, the grid was transferred onto a drop of negative stain. Aqueous 1% phosphotungstic acid solution was used as a negative stain. The sample was air dried at room temperature and finally the samples were examined by TEM (Model JEM-2100F, JEOL, Peabody, MA, USA).
2.4. In Vitro Release Study
The in vitro release study was performed using a modified Franz diffusion cell apparatus consisting of donor and receptor chambers (NK Laboratories Co., Ltd., Bangkok, Thailand). These two chambers were separated by a semipermeable membrane (MWCO 12,000–14,000 Da). The membrane was presoaked overnight in the receptor phase consisting of phosphate-buffered saline (PBS, pH 7.4). The receptor phase was degassed to remove dissolved air before being placed in the receptor chamber. The sample (1.5 mL) of each niosomal formulation was placed in the donor chamber. The receptor phase was continuously stirred at 150 rpm throughout the experiment and a controlled temperature was maintained at 34 ± 1 °C by a thermostated circulating bath (GRANT W6, Akribis Scientific Limited, Cheshire, UK). A 150 μL aliquot of the receptor medium was withdrawn at timed intervals and replaced immediately with an equal volume of fresh receptor phase. The FOS content in the receptor medium was determined using HPLC and the amount of cumulative drug release was calculated. Each formulation was performed in triplicate.
2.5. Ex Vivo Permeation Study
The ex vivo permeation study was performed across the cornea and sclera of porcine eyes obtained within 4 h after the death of pigs from a slaughterhouse. In this study, the cornea and sclera were dissected from porcine eyes and replaced with the semipermeable cellophane membrane as previously described in in vitro release study. The selected FOS-loaded Span
® 60-niosomal formulations and an aqueous saturated solution of FOS/γCD complex used as a control were conducted at least in triplicate. The FOS content in the receptor phase at timed intervals was determined using HPLC. The steady state flux was calculated as the slope of linear section of the amount of drug in the receptor chamber (
q) versus time (
t) profiles, and the apparent permeability coefficient (
Papp) was calculated from the flux (
J) according to Equation (2):
where
A is the surface area of the mounted membrane (1.7 cm
2) and
Cd is the initial concentration of the drug in the donor chamber.
2.6. Cell Viability and Short Time Exposure (STE) Test
In vitro cytotoxicity test was determined using the methylthiazolyl-diphenyl-tetrazolium bromide (MTT) assay [
27,
28]. Briefly, the niosomal formulations containing FOS without and with γCD (Sp-DCP and Sp-DCP+γCD, respectively) including their respective blank samples, i.e., B-Sp-DCP and B-Sp-DCP+γCD were evaluated for their toxicity to the rabbit corneal fibroblasts, i.e., the SIRC (rabbit corneal cell line) cells (CCL-60; ATCC, Manassas, VA, USA). Each sample was diluted to the concentration of 0.5, 1, 2, 5 and 10% (
v/v) of the test samples by a complete medium that contained Eagle’s Minimum Essential Medium and fetal bovine serum (FBS). FOS concentrations in the tested samples ranged from 0.005 to 0.1%
w/v. The cells were cultured in the complete medium and maintained at 37 °C under 5% CO
2 atmosphere. They were seeded in 96-well plates with a density of 1 × 10
5 cells/well/100 µL and incubated for 24 h. Thereafter, each test sample (100 µL) was added to the well. The cells were incubated for 24 h and washed twice with PBS (pH 7.4) at the end of incubation period. MTT solution in PBS (pH 7.4) was added to each well and incubated for 4 h. The formazan crystals were dissolved using 0.04 M HCl in isopropanol (100 µL/well). The optical density (OD) of each well was measured at 570 nm by a microplate reader (Fluostar Omega, BMG Labtech, Ortenberg, Germany). The experiments were performed in four replications, and cell viability (CV) was calculated following Equation (3). The test samples were considered to be toxic to the cells if the CV (%) was less than 70%.
where the
ODsample and
ODcontrol are an
OD of the media from the wells containing the SIRC cells incubated with the samples and MTT solution, and an
OD of media from the wells containing the cells incubated with MTT solution without the samples, respectively.
The eye irritation potential of those test samples was further evaluated based on the MTT reduction assay [
29]. The in vitro eye irritation test was performed according to the procedure of the STE test proposed by Takahashi et al. (2008) [
30]. The CV of SIRC cells was determined after they were exposed to 200 µL of either 5% or 0.05% of the test samples dispersed in normal saline for 5 min. The eye irritation potential from the STE test was scored following the criteria for STE irritation scoring. Then, the obtained scores from the 5% and the 0.05% tests were summed up to rank the eye irritation potential. The total scores were ranked as 1, 2 and 3, defined as minimal ocular irritant, moderate ocular irritant, and severe ocular irritant, respectively.
2.7. Physical and Chemical Stability Studies
To investigate the effect of γCD on stability of FOS in niosomal vesicles, selected optimal FOS-loaded niosomal formulations (in the presence and absence of γCD) and aqueous solution of FOS/γCD complex (as a control) were evaluated using the ongoing stability program following International Conference on Harmonization (ICH) guidelines [
31]. The samples were stored in tightly closed glass vials at 4 °C, long term condition (30 ± 2 °C, 75 ± 5% relative humidity (RH)) and accelerated condition (40 ± 2 °C, 75 ± 5% RH). Physical appearance was assessed, and formulations were analyzed with respect to pH, particle size and size distribution, zeta potential and the FOS content at timed intervals of 0, 1, 3 and 6 months.
2.8. Statistical Analysis
All quantitative data were presented as means ± standard deviation (SD). The data were statistically calculated using one-way ANOVA (SPSS Software, Version 16.0, SPSS Inc., Chicago, IL, USA). The p < 0.05 was considered statistically significant.


 ) Sp-DCP and (
) Sp-DCP and (  ) Sp-DCP+γCD, and, (
) Sp-DCP+γCD, and, (  ) blank Sp-DCP and (
) blank Sp-DCP and (  ) blank Sp-DCP+γCD, at various concentrations in the SIRC cells (n = 4, mean ± SD).
) blank Sp-DCP+γCD, at various concentrations in the SIRC cells (n = 4, mean ± SD).
 ) Sp-DCP and (
) Sp-DCP and (  ) Sp-DCP+γCD, and, (
) Sp-DCP+γCD, and, (  ) blank Sp-DCP and (
) blank Sp-DCP and (  ) blank Sp-DCP+γCD, at various concentrations in the SIRC cells (n = 4, mean ± SD).
) blank Sp-DCP+γCD, at various concentrations in the SIRC cells (n = 4, mean ± SD).