Novel Utilization of Therapeutic Coatings Based on Infiltrated Encapsulated Rose Bengal Microspheres in Porous Titanium for Implant Applications
Abstract
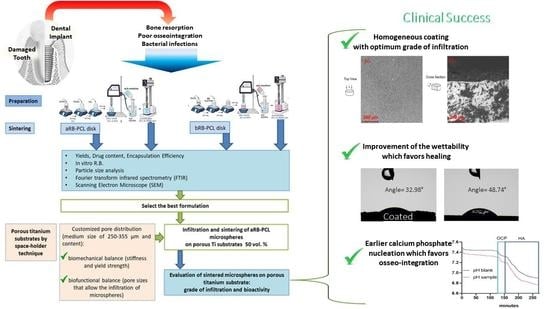
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RB-PCL Microspheres
2.3. Preparation of Disks of RB-PCL Sintered Microspheres
2.4. Yield, Drug Content, and Encapsulation Efficiency of RB-PCL Microspheres
2.5. Physical and Chemical Characterization of Microspheres
2.6. Fabrication and Basic Characterization of Porous Titanium Substrate
2.7. Coating and Infiltration of the Microspheres in the Porous Titanium Substrate
2.8. Morphological Analysis by SEM
2.9. Osteoactivity Evaluation of Sintered Microspheres by the Use of Wettability
2.10. Calcium Phosphate Nucleation In Vitro Test
2.11. Statistical Analysis
3. Results and Discussion
3.1. Manufacturing and Characterization of Microspheres and Sintered Disks
3.1.1. Comparison of the Two Processes
3.1.2. Size and Morphology
3.1.3. FTIR Spectroscopy
3.1.4. In Vitro Drug Release Studies
3.2. Characterization of the Porous c.p. Ti Substrates
Wettability of Porous Titanium Substrates
3.3. Infiltration of Porous Titanium Substrate and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcudia, A.; Begines, B.; Rodriguez-Lejarraga, P.; Greyer, V.; Godinho, V.C.F.; Pajuelo, E.; Torres, Y. Development of porous silver nanoparticle/polycaprolactone/polyvinyl alcohol coatings for prophylaxis in titanium interconnected samples for dental implants. Colloids Interface Sci. Commun. 2022, 48, 100621. [Google Scholar] [CrossRef]
- Beltran, A.M.; Begines, B.; Alcudia Cruz, A.; Rodríguez-Ortiz, J.A.; Torres, Y. Biofunctional and Tribomechanical Behavior of Porous Titanium Substrates Coated with a Bioactive Glass Bilayer (45S5–1393). ACS Appl. Mater. Interfaces 2020, 12, 30170–30180. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Macedo-de-Sousa, B.; López-Valverde, A.; Ramírez, J.M. Effectiveness of Antibacterial Surfaces in Osseointegration of Titanium Dental Implants: A Systematic Review. Antibiotics 2021, 10, 360. [Google Scholar] [CrossRef]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings 2020, 10, 568. [Google Scholar] [CrossRef]
- Nimbalkar, S.; Dhatrak, P.; Gherde, C.; Joshi, S. A review article on factors affecting bone loss in dental implants. Mater. Today Proc. 2020, 43, 970–976. [Google Scholar] [CrossRef]
- Annibali, S.; Ripari, M.; LA Monaca, G.; Tonoli, F.; Cristalli, M.P. Local complications in dental implant surgery: Prevention and treatment. Oral Implantol. 2008, 1, 21–33. [Google Scholar]
- Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E.; Gay-Escoda, C. Postoperative Infections After Dental Implant Placement: Prevalence, Clinical Features, and Treatment. Implant Dent. 2015, 24, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human peri-implantitis microbiota. Clin. Oral Implant. Res. 2014, 25, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.S.; Paterson, D.L. Antibiotics in the clinical pipeline in October 2019. J. Antibiot. 2020, 73, 329–364. [Google Scholar] [CrossRef] [PubMed]
- Demartis, S.; Rassu, G.; Murgia, S.; Casula, L.; Giunchedi, P.; Gavini, E. Improving Dermal Delivery of Rose Bengal by Deformable Lipid Nanovesicles for Topical Treatment of Melanoma. Mol. Pharm. 2021, 18, 4046–4057. [Google Scholar] [CrossRef] [PubMed]
- Demartis, S.; Obinu, A.; Gavini, E.; Giunchedi, P.; Rassu, G. Nanotechnology-based rose Bengal: A broad-spectrum biomedical tool. Dye. Pigment. 2021, 188, 109236. [Google Scholar] [CrossRef]
- Kurosu, M.; Mitachi, K.; Yang, J.; Pershing, E.V.; Horowitz, B.D.; Wachter, E.A.; Lacey, J.W., 3rd; Ji, Y.; Rodrigues, D.J. Antibacterial Activity of Pharmaceutical-Grade Rose Bengal: An Application of a Synthetic Dye in Antibacterial Therapies. Molecules 2022, 27, 322. [Google Scholar] [CrossRef]
- Bombeccari, G.P.; Guzzi, G.; Gualini, F.; Gualini, S.; Santoro, F.; Spadari, F. Photodynamic therapy to treat periimplantitis. Implant Dent. 2013, 22, 631–638. [Google Scholar] [CrossRef]
- Garcia de Carvalho, G.; Sanchez-Puetate, J.C.; Casalle, N.; Marcantonio Junior, E.; Leal Zandim-Barcelos, D. Antimicrobial photodynamic therapy associated with bone regeneration for peri-implantitis treatment: A case report. Photodiagnosis Photodyn. Ther. 2020, 30, 101705. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine 2014, 10, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Hossain, K.M.Z.; Patel, U.; Ahmed, I. Development of microspheres for biomedical applications: A review. Prog. Biomater. 2015, 4, 1–19. [Google Scholar] [CrossRef]
- Zirak, N.; Maadani, A.M.; Salahinejad, E.; Abbasnezhad, N.; Shirinbayan, M. Fabrication, drug delivery kinetics and cell viability assay of PLGA-coated vancomycin-loaded silicate porous microspheres. Ceram. Int. 2022, 48, 48–54. [Google Scholar] [CrossRef]
- Xiao, D.; Liu, Q.; Wang, D.; Xie, T.; Guo, T.; Duan, K.; Weng, J. Room-temperature attachment of PLGA microspheres to titanium surfaces for implant-based drug release. Appl. Surf. Sci. 2014, 309, 112–118. [Google Scholar] [CrossRef]
- Dawes, G.J.S.; Fratila-Apachitei, L.E.; Necula, B.S.; Apachitei, I.; Witkamp, G.J.; Duszczyk, J. Release of PLGA-encapsulated dexamethasone from microsphere loaded porous surfaces. J. Mater. Sci. Mater. Med. 2010, 21, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulia, K.; Witkamp, G.-J.; Dawes, G.J.S.; Fratila-Apachitei, L.E.; Apachitei, I.; Duszczyk, J.; Pellikaan, H. Drug release from PLGA microspheres attached to solids using supercritical CO2. J. Biomater. Appl. 2011, 25, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Coccoli, V.; Orsi, S.; Ambrosio, L.; Netti, P.A. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials 2008, 29, 4800–4807. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Stylios, G.K.; Giannoudi, M.; Giannoudis, P.V. Investigating a new drug delivery nano composite membrane system based on PVA/PCL and PVA/HA(PEG) for the controlled release of biopharmaceuticals for bone infections. Injury 2015, 46 (Suppl. S8), S39–S43. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Khosla, J.K.; Gupta, P.N.; Gupta, M. Polycaprolactone-based materials in wound healing applications. Polym. Bull. 2021, 1–23. [Google Scholar] [CrossRef]
- Manivasagam, G.; Reddy, A.; Sen, D.; Nayak, S.; Mathew, M.T.; Rajamanikam, A. Dentistry: Restorative and regenerative approaches. In Encyclopedia of Biomedical Engineering; Hosseinpour, S., Walsh, L.J., Moharamzadeh, K., Eds.; Elsevier: Oxford, UK, 2019; pp. 332–347. ISBN 978-0-12-805144-3. [Google Scholar]
- Torres, Y.; Begines, B.; Beltrán, A.M.; Boccaccini, A.R. Deposition of bioactive gelatin coatings on porous titanium: Influence of processing parameters, size and pore morphology. Surf. Coat. Technol. 2021, 421, 127366. [Google Scholar] [CrossRef]
- Torres, Y.; Trueba, P.; Pavón, J.; Montealegre, I.; Rodríguez-Ortiz, J.A. Designing, processing and characterisation of titanium cylinders with graded porosity: An alternative to stress-shielding solutions. Mater. Des. 2014, 63, 316–324. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Ternero, F.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; Montealegre-Meléndez, I.; Arévalo, C.; García-Moreno, F.; Torres, Y. Improvement of the balance between a reduced stress shielding and bone ingrowth by bioactive coatings onto porous titanium substrates. Surf. Coat. Technol. 2018, 338, 32–37. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Ternero, F.; Rodríguez-Ortiz, J.A.; Heise, S.; Boccaccini, A.R.; Lebrato, J.; Torres, Y. Bioactive coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 349, 584–592. [Google Scholar] [CrossRef]
- kakhki, R.M.; Nejati-Yazdinejad, M.; Kakeh, F. Extraction and determination of Rose Bengal in water samples by dispersive liquid–liquid microextraction coupled to UV–Vis spectrophotometry. Arab. J. Chem. 2017, 10, S2518–S2522. [Google Scholar] [CrossRef] [Green Version]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release Off. J. Control. Release Soc. 2015, 201, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassu, G.; Nieddu, M.; Bosi, P.; Trevisi, P.; Colombo, M.; Priori, D.; Manconi, P.; Giunchedi, P.; Gavini, E.; Boatto, G. Encapsulation and modified-release of thymol from oral microparticles as adjuvant or substitute to current medications. Phytomedicine 2014, 21, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Tice, T.R.; Gilley, R.M.; Eldridge, J.H.; Staas, J.K. Composition for Delivering Bioactive Agents for Immune Response and Its Preparation. U.S. Patent No 6,024,983, 15 February 2000. [Google Scholar]
- Bottino, M.C.; Münchow, E.A.; Albuquerque, M.T.P.; Kamocki, K.; Shahi, R.; Gregory, R.L.; Chu, T.-M.G.; Pankajakshan, D. Tetracycline-incorporated polymer nanofibers as a potential dental implant surface modifier. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Green, F.J. The Sigma-Aldrich Handbook of Stains, Dyes and Indicators; Sigma-Aldrich Corp: Milwaukee, WS, USA, 1991; ISBN 978-0-941633-22-2. [Google Scholar]
- Lascano, S.; Arévalo, C.; Montealegre-Melendez, I.; Muñoz, S.; Rodriguez-Ortiz, J.A.; Trueba, P.; Torres, Y. Porous Titanium for Biomedical Applications: Evaluation of the Conventional Powder Metallurgy Frontier and Space-Holder Technique. Appl. Sci. 2019, 9, 982. [Google Scholar] [CrossRef] [Green Version]
- Torres, Y.; Rodríguez, J.A.; Arias, S.; Echeverry, M.; Robledo, S.; Amigo, V.; Pavón, J.J. Processing, characterization and biological testing of porous titanium obtained by space-holder technique. J. Mater. Sci. 2012, 47, 6565–6576. [Google Scholar] [CrossRef]
- Torres, Y.; Lascano, S.; Bris, J.; Pavón, J.; Rodriguez, J.A. Development of porous titanium for biomedical applications: A comparison between loose sintering and space-holder techniques. Mater. Sci. Eng. C 2014, 37, 148–155. [Google Scholar] [CrossRef]
- Muñoz, S.; Pavón, J.; Rodríguez-Ortiz, J.A.; Civantos, A.; Allain, J.P.; Torres, Y. On the influence of space holder in the development of porous titanium implants: Mechanical, computational and biological evaluation. Mater. Charact. 2015, 108, 68–78. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Trueba, P.; Borie, F.; Alcudia, A.; Begines, B.; Rodriguez-Ortiz, J.A.; Torres, Y. Bioactive Bilayer Glass Coating on Porous Titanium Substrates with Enhanced Biofunctional and Tribomechanical Behavior. Coatings 2022, 12, 245. [Google Scholar] [CrossRef]
- ASTM C373-14(2014); Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products, Ceramic Tiles, and Glass Tiles. ASTM International: West Conshohocken, PA, USA, 2014.
- Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early osseointegration driven by the surface chemistry and wettability of dental implants. J. Appl. Oral Sci. 2015, 23, 279–287. [Google Scholar] [CrossRef]
- Zhao, W.; Michalik, D.; Ferguson, S.; Hofstetter, W.; Lemaître, J.; von Rechenberg, B.; Bowen, P. Rapid evaluation of bioactive Ti-based surfaces using an in vitro titration method. Nat. Commun. 2019, 10, 2062. [Google Scholar] [CrossRef] [Green Version]
- Gorodzha, S.N.; Surmeneva, M.A.; Surmenev, R.A. Fabrication and characterization of polycaprolactone cross- linked and highly-aligned 3-D artificial scaffolds for bone tissue regeneration via electrospinning technology. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012024. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Thakur, N.S.; Chandna, S.; Bhaumik, J. Development of agri-biomass based lignin derived zinc oxide nanocomposites as promising UV protectant-cum-antimicrobial agents. J. Mater. Chem. B 2020, 8, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Cossu, M.; Langasco, R.; Carta, A.; Cavalli, R.; Giunchedi, P.; Gavini, E. Propolis as lipid bioactive nano-carrier for topical nasal drug delivery. Colloids Surf. B. Biointerfaces 2015, 136, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Pokrowiecki, R. The paradigm shift for drug delivery systems for oral and maxillofacial implants. Drug Deliv. 2018, 25, 1504–1515. [Google Scholar] [CrossRef] [Green Version]
- Civantos, A.; Domínguez, C.; Pino, R.J.; Setti, G.; Pavón, J.J.; Martínez-Campos, E.; Garcia Garcia, F.J.; Rodríguez, J.A.; Allain, J.P.; Torres, Y. Designing bioactive porous titanium interfaces to balance mechanical properties and in vitro cells behavior towards increased osseointegration. Surf. Coat. Technol. 2019, 368, 162–174. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Civantos, A.; Dominguez-Trujillo, C.; Moriche, R.; Rodríguez-Ortiz, J.A.; García-Moreno, F.; Webster, T.J.; Kamm, P.H.; Restrepo, A.M.; Torres, Y. Porous titanium surfaces to control bacteria growth: Mechanical properties and sulfonated polyetheretherketone coatings as antibiofouling approaches. Metals 2019, 9, 995. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Shi, J.; Li, W.; Sun, K. Morphology, Wettability, and Mechanical Properties of Polycaprolactone/Hydroxyapatite Composite Scaffolds With Interconnected Pore Structures Fabricated by a Mini-Deposition System. Polym. Eng. Sci. 2012, 52, 2396–2402. [Google Scholar] [CrossRef]
- Civantos, A.; Giner, M.; Trueba, P.; Lascano, S.; Montoya-García, M.-J.; Arévalo, C.; Vázquez, M.Á.; Allain, J.P.; Torres, Y. In Vitro Bone Cell Behavior on Porous Titanium Samples: Influence of Porosity by Loose Sintering and Space Holder Techniques. Metals 2020, 10, 696. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Nishiguchi, S.; Nakamura, T.; Kobayashi, M.; Kim, H.-M.; Miyaji, F.; Kokubo, T. The effect of heat treatment on bone-bonding ability of alkali-treated titanium. Biomaterials 1999, 20, 491–500. [Google Scholar] [CrossRef]












| RB Content in w1 (mg/mL) | w1 Media | pH w1 Media | PCL Content in o (CH2Cl2) (mg/mL) | w2 Media | w1/o/w2 Ratio (v/v/v) | |
|---|---|---|---|---|---|---|
| aRB-PCL | 5.4 | 0.1 M HCl in H2O | 1 | 60 | 0.1 M HCl 0.5% PVA in H2O | 0.5/4.5/125 |
| bRB-PCL | 5.4 | 0.5% PVA in H2O | 6.9 | 60 | 0.1 M HCl 0.5% PVA in H2O | 0.5/4.5/125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accioni, F.; Rassu, G.; Begines, B.; Rodríguez-Albelo, L.M.; Torres, Y.; Alcudia, A.; Gavini, E. Novel Utilization of Therapeutic Coatings Based on Infiltrated Encapsulated Rose Bengal Microspheres in Porous Titanium for Implant Applications. Pharmaceutics 2022, 14, 1244. https://doi.org/10.3390/pharmaceutics14061244
Accioni F, Rassu G, Begines B, Rodríguez-Albelo LM, Torres Y, Alcudia A, Gavini E. Novel Utilization of Therapeutic Coatings Based on Infiltrated Encapsulated Rose Bengal Microspheres in Porous Titanium for Implant Applications. Pharmaceutics. 2022; 14(6):1244. https://doi.org/10.3390/pharmaceutics14061244
Chicago/Turabian StyleAccioni, Francesca, Giovanna Rassu, Belén Begines, Luisa Marleny Rodríguez-Albelo, Yadir Torres, Ana Alcudia, and Elisabetta Gavini. 2022. "Novel Utilization of Therapeutic Coatings Based on Infiltrated Encapsulated Rose Bengal Microspheres in Porous Titanium for Implant Applications" Pharmaceutics 14, no. 6: 1244. https://doi.org/10.3390/pharmaceutics14061244