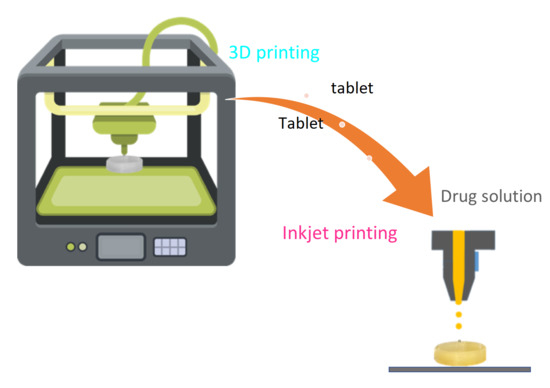
Coupling of Fused Deposition Modeling and Inkjet Printing to Produce Drug Loaded 3D Printed Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetric Analysis (TGA)
2.3. Differential Scanning Calorimetry (DSC)
2.4. X-ray Powder Diffraction (XRPD)
2.5. Design and 3D Printing of the Plain PVA Tablets
2.6. Evaluation of Printing Reproducibility
2.7. Deposition of Minoxidil Solution by Inkjet Printing
2.8. Dissolution Study and Ultraviolet-Visible (UV–VIS) Spectroscopy Analysis
2.9. Determination of Minoxidil
2.10. Weight Variation
2.11. Friability
2.12. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Thermal Characterization
3.2. X-ray Powder Diffraction (XRPD)
3.3. 3D Printing of the Plain PVA Tablets
3.4. Friability
3.5. Deposition of Minoxidil Solution by Inkjet Printing
3.6. Dissolution Study
3.7. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horas, K.; Hoffmann, R.; Faulenbach, M.; Heinz, S.M.; Langheinrich, A.; Schweigkofler, U. Advances in the Preoperative Planning of Revision Trauma Surgery Using 3D Printing Technology. J. Orthop. Trauma 2020, 34, e181–e186. [Google Scholar] [CrossRef]
- Alturkistani, R.; Kavin, A.; Devasahayam, S.; Thomas, R.; Colombini, E.L.; Cifuentes, C.A.; Homer-Vanniasinkam, S.; Wurdemann, H.A.; Moazen, M. Affordable passive 3D-printed prosthesis for persons with partial hand amputation. Prosthet. Orthot. Int. 2020, 44, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Clifton, W.; Damon, A.; Soares, C.; Nottmeier, E.; Pichelmann, M. Investigation of a Three-Dimensional Printed Dynamic Cervical Spine Model for Anatomy and Physiology Education. Clin. Anat. 2021, 34, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Grottkau, B.E.; He, Z.; Shu, L.; Yang, L.; Ma, M.; Ye, C. Biofabrication of valentine-shaped heart with a composite hydrogel and sacrificial material. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110205. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 2015, 7, 045012. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Tabriz, A.G.; Nandi, U.; Hurt, A.P.; Hui, H.W.; Karki, S.; Gong, Y.; Kumar, S.; Douroumis, D. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment. Int. J. Pharm. 2021, 593, 20147. [Google Scholar] [CrossRef]
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive Manufacturing of Personalized Pharmaceutical Dosage Forms via Stereolithography. Pharmaceutics 2019, 11, 645. [Google Scholar] [CrossRef] [Green Version]
- Dumpa, R.N.; Bandari, S.; Repka, M.A. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176–198. [Google Scholar] [CrossRef] [Green Version]
- Paul, G.M. Medical Applications for 3D Printing: Recent Developments. Mo. Med. 2018, 115, 75–81. [Google Scholar]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control Release 2017, 261, 207–215. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Adibkia, K. Made-on-demand, complex and personalized 3D-printed drug products. Bioimpacts 2018, 8, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Govender, R.; Abrahmsén-Alami, S.; Folestad, S.; Larsson, A. High Content Solid Dispersions for Dose Window Extension: A basis for design flexibility in fused deposition modelling. Pharm. Res. 2019, 37, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karalia, D.; Siamidi, A.; Karalis, V.; Vlachou, M. 3D-Printed Oral Dosage Forms: Mechanical Properties, Computational Approaches and Applications. Pharmaceutics 2021, 13, 1401. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Kissi, E.O.; Larsson, A.; Tho, I. Polymers in pharmaceutical additive manufacturing: A balancing act between printability and product performance. Adv. Drug Deliv. Rev. 2021, 177, 113923. [Google Scholar] [CrossRef]
- Rahim, T.N.; Abdullah, A.M.; Akil, H.M. Recent Developments in Fused Deposition Modeling-Based 3D Printing of Polymers and Their Composites. Polym. Rev. 2019, 59, 589–624. [Google Scholar] [CrossRef]
- Saviano, M.; Aquino, R.P.; Del Gaudio, P.; Sansone, F.; Russo, P. Poly (vinyl alcohol) 3D printed tablets: The effect of polymer particle size on drug loading and process efficiency. Int. J. Pharm. 2019, 561, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D Printing Factors Important for the Fabrication of Polyvinylalcohol Filament-Based Tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Kobayashi, M.; Martínez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of PVA-based caplets. Int. J. Pharm. 2016, 514, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, J.; Wang, M.; Wang, L.; Yang, J. 3D Printed Polyvinyl Alcohol Tablets with Multiple Release Profiles. Sci. Rep. 2019, 9, 12487. [Google Scholar] [CrossRef]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D Printed Medicines: Optimising material properties for successful passive diffusion loading of filaments for fused deposition modelling of solid dosage forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [Green Version]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which techniques and polymers are more successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A holistic materials process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Barnes, M.; McMillin, R.; Cook, D.W.; Smith, S.; Halquist, M.; Wijesinghe, D.; Roper, T.D. 3D Printing of Metformin HCl PVA Tablets by Fused Deposition Modeling: Drug loading, tablet design, and dissolution studies. Aaps Pharmscitech 2019, 20, 195–206. [Google Scholar] [CrossRef]
- Junqueira, L.A.; Raposo, F.J.; Vaz, U.P.; Brandão, M.A.F.; Raposo, N.R.B. Fabrication Of Oral Low-Dose Minoxidil Printlets Using A Novel Single-Step Process. J. Multidiscip. Eng. Sci. Technol. 2019, 6, 10466–10470. [Google Scholar]
- Fan, J.; Men, Y.; Tseng, K.H.; Ding, Y.; Ding, Y.; Villarreal, F.; Tan, C.; Li, B.; Pan, T. Dotette: Programmable, high-precision, plug-and-play droplet pipetting. Biomicrofluidics 2018, 12, 034107. [Google Scholar] [CrossRef] [PubMed]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef] [PubMed]
- Size, F.D.A. Shape, and Other Physical Attributes of Generic Tablets and Capsules: Guidance for Industry. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm377938.pdf (accessed on 15 August 2021).
- Hong, X.; Zou, L.; Zhao, J.; Li, C.; Cong, L. Dry-wet spinning of PVA fiber with high strength and high Young’s modulus. IOP Conf. Ser. Mater. Sci. Eng. 2018, 439, 042011. [Google Scholar] [CrossRef] [Green Version]
- Betti, N.A. Thermogravimetric Analysis on PVA/PVP Blend Under Air Atmosphere. Eng. Technol. J. 2016, 34, 2433–2441. [Google Scholar]
- Nukala, P.K.; Palekar, S.; Solanki, N.; Fu, Y.; Patki, M.; Shohatee, A.A.; Trombetta, L.; Patel, K. Investigating the application of fused deposition modeling 3D printing pattern in preparation of patient-tailored dosage forms. J. 3D Print. Med. 2019, 3, 1–16. [Google Scholar] [CrossRef]
- Pereira, M.N.; Schulte, H.L.; Duarte, N.; Lima, E.M.; Sá-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M.S. Solid effervescent formulations as new approach for topical minoxidil delivery. Eur. J. Pharm. Sci. 2017, 96, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Ahad, N.; Saion, E.; Gharibshahi, E. Structural, Thermal, and Electrical Properties of PVA-Sodium Salicylate Solid Composite Polymer Electrolyte. J. Nanomater. 2012, 2012, 857569. [Google Scholar] [CrossRef] [Green Version]
- Bhargav, P.B.; Mohan, V.M.; Sharma, A.K.; Rao, V. Structural, Electrical and Optical Characterization of Pure and Doped Poly (Vinyl Alcohol) (PVA) Polymer Electrolyte Films. Int. J. Polym. Mater. 2007, 56, 579–591. [Google Scholar] [CrossRef]
- Brambilla, C.R.M.; Okafor-Muo, O.L.; Hassanin, H.; ElShaer, A. 3DP Printing of Oral Solid Formulations: A Systematic Review. Pharmaceutics 2021, 13, 358. [Google Scholar] [CrossRef]
- Abaci, A.; Gedeon, C.; Kuna, A.; Guvendiren, M. Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. The United States Pharmacopeia and National Formulary USP 41–NF 36; The United States Pharmacoepeial Convention, Inc.: Rockville, MD, USA, 2018. [Google Scholar]
- Sharma, V.; Shaik, K.M.; Choudhury, A.; Kumar, P.; Kala, P.; Sultana, Y.; Shukla, R.; Kumar, D. Investigations of process parameters during dissolution studies of drug loaded 3D printed tablets. Proc. Inst. Mech. Eng. H 2021, 235, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. Aaps Pharmscitech 2018, 19, 3403–3413. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.; Scoutaris, N.; Lamprou, D.; Mallinson, D.; Douroumis, D. Inkjet printing of insulin microneedles for transdermal delivery. Drug Deliv. Transl. Res. 2015, 5, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Scoutaris, N.; Chai, F.; Maurel, B.; Sobocinski, J.; Zhao, M.; Moffa, J.; Craig, D.; Martel, B.; Blanchemain, N.; Douroumis, D. Development and Biological Evaluation of Inkjet Printed Drug Coatings on Intravascular Stent. Mol. Pharm. 2016, 13, 125–133. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [Green Version]
- Randolph, M.; Tosti, A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Dermatol. 2021, 84, 737–746. [Google Scholar] [CrossRef]
- Nascimento, I.; Harries, M.; Rocha, V.B.; Thompson, J.Y.; Wong, C.H.; Varkaneh, H.K.; Guimarães, N.S.; Rocha Arantes, A.J.; Marcolino, M.S. Effect of Oral Minoxidil for Alopecia: Systematic Review. Int. J. Trichol. 2020, 12, 147–155. [Google Scholar] [CrossRef]
- Jimenez-Cauhe, J.; Saceda-Corralo, D.; Rodrigues-Barata, R.; Hermosa-Gelbard, A.; Moreno-Arrones, O.M.; Fernandez-Nieto, D.; Vaño-Galvan, S. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J. Am. Acad. Dermatol. 2019, 81, 648–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, E.; Sinclair, R. Treatment of chronic telogen effluvium with oral minoxidil: A retrospective study. F1000Res 2017, 6, 1650. [Google Scholar] [CrossRef]
- Villani, A.; Fabbrocini, G.; Ocampo-Candiani, J.; Ruggiero, A.; Ocampo-Garza, S.S. Review of oral minoxidil as treatment of hair disorders: In search of the perfect dose. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Soh, S. Printing Tablets with Fully Customizable Release Profiles for Personalized Medicine. Adv. Mater. 2015, 27, 7847–7853. [Google Scholar] [CrossRef]
- Tagami, T.; Kuwata, E.; Sakai, N.; Ozeki, T. Drug Incorporation into Polymer Filament Using Simple Soaking Method for Tablet Preparation Using Fused Deposition Modeling. Biol. Pharm. Bull. 2019, 42, 1753–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Parameter | Diameter (mm) | Height (mm) | Depth (mm) |
|---|---|---|---|
| Mean | 12.00 | 3.00 | 2.01 |
| SD | 0.09 | 0.02 | 0.02 |
| RSD | 0.74 | 0.70 | 0.94 |
| P70 | P100 | |||
|---|---|---|---|---|
| Tablet Weight (mg) | Deviation % | Tablet Weight (mg) | Deviation % | |
| Average | 238.9 | 0.00 | 234.9 | 0.00 |
| Median | 236.3 | −1.09 | 235.7 | 0.33 |
| Maximum | 247.5 | 3.60 | 242.9 | 3.41 |
| Minimum | 233.5 | −2.26 | 228.2 | −2.85 |
| SD | 4.92 | 2.06 | 4.63 | 1.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junqueira, L.A.; Tabriz, A.G.; Raposo, F.J.; Carobini, L.R.; Vaz, U.P.; Brandão, M.A.F.; Douroumis, D.; Raposo, N.R.B. Coupling of Fused Deposition Modeling and Inkjet Printing to Produce Drug Loaded 3D Printed Tablets. Pharmaceutics 2022, 14, 159. https://doi.org/10.3390/pharmaceutics14010159
Junqueira LA, Tabriz AG, Raposo FJ, Carobini LR, Vaz UP, Brandão MAF, Douroumis D, Raposo NRB. Coupling of Fused Deposition Modeling and Inkjet Printing to Produce Drug Loaded 3D Printed Tablets. Pharmaceutics. 2022; 14(1):159. https://doi.org/10.3390/pharmaceutics14010159
Chicago/Turabian StyleJunqueira, Laura Andrade, Atabak Ghanizadeh Tabriz, Francisco José Raposo, Luana Rocha Carobini, Urias Pardócimo Vaz, Marcos Antônio Fernandes Brandão, Dennis Douroumis, and Nádia Rezende Barbosa Raposo. 2022. "Coupling of Fused Deposition Modeling and Inkjet Printing to Produce Drug Loaded 3D Printed Tablets" Pharmaceutics 14, no. 1: 159. https://doi.org/10.3390/pharmaceutics14010159