Stimuli-Responsive Polymers for Transdermal, Transmucosal and Ocular Drug Delivery
Abstract
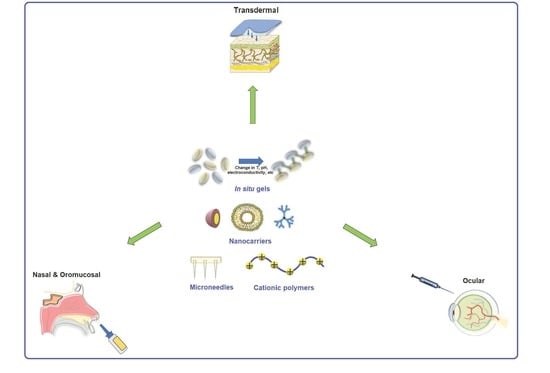
:1. Introduction
2. Transdermal Drug Delivery Systems
2.1. Skin Barrier
2.2. Approaches to Overcome the Skin Barrier
3. Transmucosal Drug Delivery Based on Stimuli-Responsive Polymers
3.1. Benefits and Limitations Associated with Nasal Drug Delivery
3.2. Approaches to Enhance Nasal Drug Delivery by Using Smart Polymers
3.3. Oromucosal Drug Delivery Based on Smart Polymers
4. Ocular Drug Delivery Systems
4.1. Ocular Barriers
4.2. Polymeric Stimuli-Responsive Ocular DDSs
4.2.1. Polymeric Thermosensitive DDSs
4.2.2. Polymeric pH-Sensitive DDSs
4.2.3. DDSs Based on Ionic Strength-Sensitive Polymers
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yao, C.; Li, Y.; Wang, Z.; Song, C.; Hu, X.; Liu, S. Cytosolic NQO1 Enzyme-Activated Near-Infrared Fluorescence Imaging and Photodynamic Therapy with Polymeric Vesicles. ACS Nano 2020, 14, 1919–1935. [Google Scholar] [CrossRef]
- Araste, F.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Self-assembled polymeric vesicles: Focus on polymersomes in cancer treatment. J. Control. Release 2020, 330, 502–528. [Google Scholar] [CrossRef]
- Barani, M.; Sargazi, S.; Hajinezhad, M.R.; Rahdar, A.; Sabir, F.; Pardakhty, A.; Zargari, F.; Anwer, M.K.; Aboudzadeh, M.A. Preparation of pH-Responsive Vesicular Deferasirox: Evidence from In Silico, In Vitro, and In Vivo Evaluations. ACS Omega 2021, 6, 24218–24232. [Google Scholar] [CrossRef]
- Bilal, M.; Qindeel, M.; Raza, A.; Mehmood, S.; Rahdar, A. Stimuli-responsive nanoliposomes as prospective nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102916. [Google Scholar] [CrossRef]
- Gu, M.; Wang, X.; Toh, T.B.; Chow, E.K.-H. Applications of stimuli-responsive nanoscale drug delivery systems in translational research. Drug Discov. Today 2018, 23, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Zhang, Z.; Shah, P.J.; Dubey, P.B.; Gandhi, J.K. Intraoral and peroral drug delivery systems. In In-Vitro and In-Vivo Tools in Drug Delivery Research for Optimum Clinical Outcomes; CRC Press: Boca Raton, FL, USA, 2018; p. 25. [Google Scholar]
- Qi, J.; Lu, Y.; Wu, W.; Yuan, H. Peroral targeting of drug micro or nanocarriers to sites beyond the gastrointestinal tract. Med. Res. Rev. 2021, 41, 2590–2598. [Google Scholar]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Vlachou, M.; Siamidi, A. Biopolymers, liposomes, and nanofibers as modified peroral drug release formulants. In Nanomaterials for Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–270. [Google Scholar]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, O.; Hoogenboom, R. Drug Delivery Systems Based on Poly (2-Oxazoline) s and Poly (2-Oxazine) s. Adv. Ther. 2020, 3, 1900168. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M. Transdermal and intravenous nano drug delivery systems: Present and future. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 499–550. [Google Scholar]
- Marasini, N.; Haque, S.; Kaminskas, L.M. Polymer-drug conjugates as inhalable drug delivery systems: A review. Curr. Opin. Colloid Interface Sci. 2017, 31, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, H.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef]
- Miranda, M.; Rodrigues, M.; Domingues, R.; Torrado, E.; Reis, R.L.; Pedrosa, J.; Gomes, M.E. Exploring inhalable polymeric dry powders for anti-tuberculosis drug delivery. Mater. Sci. Eng. C 2018, 93, 1090–1103. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Song, L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: A review. J. Nanobiotechnol. 2020, 18, 1–25. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Ban, M.M.; Chakote, V.R.; Dhembre, G.N.; Rajguru, J.R.; Joshi, D.A. In-situ gel for nasal drug delivery. Int. J. Dev. Res. 2018, 8, 18763–18769. [Google Scholar]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2017, 127, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Seah, B.C.-Q.; Teo, B.M. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandl, M.; Bauer-Brandl, A. Oromucosal drug delivery: Trends in in-vitro biopharmaceutical assessment of new chemical entities and formulations. Eur. J. Pharm. Sci. 2018, 128, 112–117. [Google Scholar] [CrossRef] [Green Version]
- d’Angelo, I.; Fraix, A.; Ungaro, F.; Quaglia, F.; Miro, A. Poly (ethylene oxide)/hydroxypropyl-β-cyclodextrin films for oromucosal delivery of hydrophilic drugs. Int. J. Pharm. 2017, 531, 606–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Radeke, C.; Jacobsen, J.; Lind, J.U.; Mu, H. Multi-material 3D printing of programmable and stretchable oromucosal patches for delivery of saquinavir. Int. J. Pharm. 2021, 610, 121236. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Cunha, D.; Ferreira, D.C.; Reis, S.; Costa, P.J. Oromucosal precursors of in loco hydrogels for wound-dressing and drug delivery in oral mucositis: Retain, resist, and release. Mater. Sci. Eng. C 2020, 118, 111413. [Google Scholar] [CrossRef]
- El-Salamouni, N.S.; Hanafy, A.S. Hyaluronic-benzydamine oromucosal films outperform conventional mouth rinse in ulcer healing. J. Drug Deliv. Sci. Technol. 2021, 65, 102690. [Google Scholar] [CrossRef]
- Shirvan, A.R.; Bashari, A.; Hemmatinejad, N. New insight into the fabrication of smart mucoadhesive buccal patches as a novel controlled-drug delivery system. Eur. Polym. J. 2019, 119, 541–550. [Google Scholar] [CrossRef]
- Porfiryeva, N.; Nasibullin, S.F.; Abdullina, S.G.; Tukhbatullina, I.K.; Moustafine, R.I.; Khutoryanskiy, V.V. Acrylated Eudragit® E PO as a novel polymeric excipient with enhanced mucoadhesive properties for application in nasal drug delivery. Int. J. Pharm. 2019, 562, 241–248. [Google Scholar] [CrossRef]
- Jelkmann, M.; Leichner, C.; Zaichik, S.; Laffleur, F.; Bernkop-Schnürch, A. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int. J. Biol. Macromol. 2020, 158, 1037–1046. [Google Scholar] [CrossRef]
- Jain, A.; Hurkat, P.; Jain, A.; Jain, A.; Jain, A.; Jain, S.K. Thiolated polymers: Pharmaceutical tool in nasal drug delivery of proteins and peptides. Int. J. Pept. Res. Ther. 2019, 25, 15–26. [Google Scholar] [CrossRef]
- Udupa, N.; Chonkar, A.; Nayak, U.Y. Smart polymers in nasal drug delivery. Indian J. Pharm. Sci. 2015, 77, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Gupta, P.; Yadav, K.S. Applications of microneedles in delivering drugs for various ocular diseases. Life Sci. 2019, 237, 116907. [Google Scholar] [CrossRef]
- Singh, R.R.T.; Tekko, I.; McAvoy, K.; McMILLIAN, H.; Jones, D.; Donnelly, R. Minimally invasive microneedles for ocular drug delivery. Expert Opin. Drug Deliv. 2016, 14, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef]
- Özsoy, Y.; Güngör, S.; Kahraman, E.; Durgun, M.E. Polymeric micelles as a novel carrier for ocular drug delivery. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–117. [Google Scholar]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; Du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent Advances in the Development of In Situ Gelling Drug Delivery Systems for Non-Parenteral Administration Routes. Pharmaceutics 2020, 12, 859. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Machale, M.U.; Yadav, R.P.; Sharma, M.; Patel, A.K. Role of Transdermal Drug Delivery System. Asian J. Pharm. Res. Dev. 2021, 9, 137–143. [Google Scholar]
- Singh, S.; Verma, D.; Baghel, P.; Prasad, J. Transdermal Drug Delivery System: A Novel Drug Delivery System. Adv. J. Bioact. Mol. 2021, 2, 1–13. [Google Scholar]
- Liu, C.; Hui, M.; Quan, P.; Fang, L. Drug in adhesive patch of palonosetron: Effect of pressure sensitive adhesive on drug skin permeation and in vitro-in vivo correlation. Int. J. Pharm. 2016, 511, 1088–1097. [Google Scholar] [CrossRef]
- Nagadev, C.; Rao, M.; Venkatesh, P.; Hepcykalarani, D.; Prema, R. A Review on Transdermal Drug Delivery Systems. Asian J. Res. Pharm. Sci. 2020, 10, 109–114. [Google Scholar] [CrossRef]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.J.M.; Van Den Bogaard, E.H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2018, 71, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Anjo, T.; Kaneko, A.; Senoo, Y.; Shibata, A.; Takama, H.; Yokoyama, K.; Nishito, Y.; Ono, T.; Taya, C.; et al. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat. Commun. 2017, 8, 14609. [Google Scholar] [CrossRef]
- Kwatra, B. Collagen Supplementation: Therapy for Skin Disorders: A Review. World J. Pharm. Res. 2020, 9, 2504–2518. [Google Scholar]
- Wang, F.-Y.; Chen, Y.; Huang, Y.-Y.; Cheng, C.-M. Transdermal drug delivery systems for fighting common viral infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1498–1508. [Google Scholar] [CrossRef]
- Rahmati, M.; Blaker, J.; Lyngstadaas, S.; Mano, J.; Haugen, H. Designing multigradient biomaterials for skin regeneration. Mater. Today Adv. 2020, 5, 100051. [Google Scholar] [CrossRef]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2018, 45, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Uchechi, O.; Ogbonna, J.D.; Attama, A.A. Nanoparticles for Dermal and Transdermal Drug Delivery. Appl. Nanotechnol. Drug Deliv. 2014, 4, 193–227. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm. Sin. B 2019, 9, 902–922. [Google Scholar] [CrossRef] [PubMed]
- Elshafeey, A.H.; Hamza, Y.E.; Amin, S.Y.; Zia, H. In vitro transdermal permeation of fenoterol hydrobromide. J. Adv. Res. 2012, 3, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Otterbach, A.; Lamprecht, A. Enhanced Skin Permeation of Estradiol by Dimethyl Sulfoxide Containing Transdermal Patches. Pharmaceutics 2021, 13, 320. [Google Scholar] [CrossRef]
- Shabbir, M.; Ali, S.; Farooq, M.; Adnan, S.; Yousaf, M.; Idrees, A.; Rehman, K.; Shahid, N. Formulation Factors Affecting In Vitro and Ex Vivo Permeation of Bisoprolol Fumarate from a Matrix Transdermal Patch. Adv. Polym. Technol. 2015, 35, 237–247. [Google Scholar] [CrossRef]
- Özgüney, I.S.; Karasulu, H.Y.; Kantarci, G.; Sözer, S.; Güneri, T.; Ertan, G. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS Pharm. 2006, 7, E39–E45. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Kumar, N.; Ahad, A.; Aqil, M.; Sultana, Y. Enhanced delivery of diclofenac diethylamine loaded Eudragit RL 100® transdermal system against inflammation. J. Polym. Eng. 2015, 35, 699–708. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.Y.; Cho, H.K.; Hong, W.J.; Kim, J.; Seo, H.; Choi, I.; Lee, Y.; Kim, J.; Min, S.-J.; et al. Cell-Penetrating Peptide-Patchy Deformable Polymeric Nanovehicles with Enhanced Cellular Uptake and Transdermal Delivery. Biomacromolecules 2018, 19, 2682–2690. [Google Scholar] [CrossRef]
- Ramadon, D.; Permana, A.D.; Courtenay, A.J.; McCrudden, M.T.C.; Tekko, I.A.; McAlister, E.; Anjani, Q.K.; Utomo, E.; McCarthy, H.O.; Donnelly, R.F. Development, Evaluation, and Pharmacokinetic Assessment of Polymeric Microarray Patches for Transdermal Delivery of Vancomycin Hydrochloride. Mol. Pharm. 2020, 17, 3353–3368. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef]
- Sadeqi, A.; Nejad, H.R.; Kiaee, G.; Sonkusale, S. Cost-Effective Fabrication of Chitosan Microneedles for Transdermal Drug Delivery. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5737–5740. [Google Scholar]
- Chen, Y.; Xian, Y.; Carrier, A.J.; Youden, B.; Servos, M.; Cui, S.; Luan, T.; Lin, S.; Zhang, X. A simple and cost-effective approach to fabricate tunable length polymeric microneedle patches for controllable transdermal drug delivery. RSC Adv. 2020, 10, 15541–15546. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef]
- Yin, Z.; Kuang, D.; Wang, S.; Zheng, Z.; Yadavalli, V.K.; Lu, S. Swellable silk fibroin microneedles for transdermal drug delivery. Int. J. Biol. Macromol. 2018, 106, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Ren, J.W.; Chen, B.Z.; Jin, X.; Zhang, C.Y.; Guo, X.D. Effect of humidity on mechanical properties of dissolving microneedles for transdermal drug delivery. J. Ind. Eng. Chem. 2018, 59, 251–258. [Google Scholar] [CrossRef]
- Vora, L.; Courtenay, A.; Tekko, I.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2019, 146, 290–298. [Google Scholar] [CrossRef]
- Mao, J.; Wang, H.; Xie, Y.; Fu, Y.; Li, Y.; Liu, P.; Du, H.; Zhu, J.; Dong, L.; Hussain, M.; et al. Transdermal delivery of rapamycin with poor water-solubility by dissolving polymeric microneedles for anti-angiogenesis. J. Mater. Chem. B 2019, 8, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Ramalheiro, A.; Paris, J.L.; Silva, B.F.; Pires, L.R. Rapidly dissolving microneedles for the delivery of cubosome-like liquid crystalline nanoparticles with sustained release of rapamycin. Int. J. Pharm. 2020, 591, 119942. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Charoensumran, P.; Ajiro, H. Controlled release of testosterone by polymer-polymer interaction enriched organogel as a novel transdermal drug delivery system: Effect of limonene/PG and carbon-chain length on drug permeability. React. Funct. Polym. 2019, 148, 104461. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuang, Y.; Li, J.; Liu, X.; He, S. Poly(Acrylic Acid)-Modified MoS2 Nanoparticle-Based Transdermal Delivery of Atenolol. Int. J. Nanomed. 2020, 15, 5517–5526. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Mandal, U.K.; Al-Japairai, K.A.S.; Raman, S. Transdermal drug delivery system through polymeric microneedle: A recent update. J. Drug Deliv. Sci. Technol. 2020, 60, 101877. [Google Scholar] [CrossRef]
- Mujawar, N.; Ghatage, S.; Navale, S.; Sankpal, B.; Patil, S.; Patil, S. Nasal drug delivery: Problem solution and its application. J. Curr. Pharm. Res. 2014, 4, 1231. [Google Scholar]
- Menzel, C.; Jelkmann, M.; Laffleur, F.; Bernkop-Schnürch, A. Nasal drug delivery: Design of a novel mucoadhesive and in situ gelling polymer. Int. J. Pharm. 2017, 517, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Feridooni, T.; Agu, R.U. Novel Mucoadhesive Polymers for Nasal Drug Delivery. In Recent Advances in Novel Drug Carrier Systems; InTechOpen: Rijeka, Croacia, 2012; pp. 315–330. [Google Scholar]
- Adnet, T.; Groo, A.-C.; Picard, C.; Davis, A.; Corvaisier, S.; Since, M.; Bounoure, F.; Rochais, C.; Le Pluart, L.; Dallemagne, P.; et al. Pharmacotechnical Development of a Nasal Drug Delivery Composite Nanosystem Intended for Alzheimer’s Disease Treatment. Pharmaceutics 2020, 12, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Tao, J.; Wang, J. Design and Application in Delivery System of Intranasal Antidepressants. Front. Bioeng. Biotechnol. 2020, 8, 626882. [Google Scholar] [CrossRef]
- Kashyap, K.; Shukla, R. Drug Delivery and Targeting to the Brain Through Nasal Route: Mechanisms, Applications and Challenges. Curr. Drug Deliv. 2019, 16, 887–901. [Google Scholar] [CrossRef]
- Bruinsmann, F.A.; Vaz, G.R.; Alves, A.D.C.S.; Aguirre, T.; Pohlmann, A.R.; Guterres, S.S.; Sonvico, F. Nasal Drug Delivery of Anticancer Drugs for the Treatment of Glioblastoma: Preclinical and Clinical Trials. Molecules 2019, 24, 4312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, J.; Smyth, H.D.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- Taylor, K.M.; Aulton, M.E. Aulton’s Pharmaceutics E-Book: The Design and Manufacture of Medicines; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnürch, A. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- Abdelnabi, D.M.; Abdallah, M.H.; Elghamry, H.A. Buspirone Hydrochloride Loaded In Situ Nanovesicular Gel as an Anxiolytic Nasal Drug Delivery System: In Vitro and Animal Studies. AAPS PharmSciTech 2019, 20, 134. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Tech. Eng. 2020, 65, 243–272. [Google Scholar] [CrossRef] [Green Version]
- González, L.F.; Acuña, E.; Arellano, G.; Morales, P.; Sotomayor, P.; Oyarzun-Ampuero, F.; Naves, R. Intranasal delivery of interferon-β-loaded nanoparticles induces control of neuroinflammation in a preclinical model of multiple sclerosis: A promising simple, effective, non-invasive, and low-cost therapy. J. Control. Release 2021, 331, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, M.; Fu, Y.; Li, Y.; Gong, T.; Zhang, Z.; Sun, X. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J. Control. Release 2016, 228, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Marxen, E.; Mosgaard, M.D.; Pedersen, A.M.L.; Jacobsen, J. Mucin dispersions as a model for the oromucosal mucus layer in in vitro and ex vivo buccal permeability studies of small molecules. Eur. J. Pharm. Biopharm. 2017, 121, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.K.; Xu, Y.; Worsley, A.; Wong, I.C. Oral transmucosal drug delivery for pediatric use. Adv. Drug Deliv. Rev. 2014, 73, 50–62. [Google Scholar] [CrossRef]
- Giannola, L.I.; Sutera, F.M.; De Caro, V. Physical methods to promote drug delivery on mucosal tissues of the oral cavity. Expert Opin. Drug Deliv. 2013, 10, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, F.; Chowdhry, B.Z.; Antonijević, M.D.; Boateng, J.S. Novel films for drug delivery via the buccal mucosa using model soluble and insoluble drugs. Drug Dev. Ind. Pharm. 2011, 38, 1207–1220. [Google Scholar] [CrossRef]
- Morales, J.O.; Vuddanda, P.R.; Velaga, S. Controlled Drug Delivery via the Buccal and Sublingual Routes. In Fundamentals of Drug Delivery; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2021; pp. 433–448. [Google Scholar]
- Paderni, C.; Compilato, D.; Giannola, L.I.; Campisi, G. Oral local drug delivery and new perspectives in oral drug formulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e25–e34. [Google Scholar] [CrossRef]
- Hearnden, V.; Sankar, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv. Drug Deliv. Rev. 2011, 64, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Date, A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [Green Version]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraan, H. Polymer-based oral dissolving films for polio vaccination. In Novel Formulations and Delivery Strategies for Inactivated Polio Vaccines; Institute of Translational Vaccinology (Intravacc): Bilthoven, The Netherlands, 2018; p. 161. [Google Scholar]
- Goyal, A.K.; Singh, R.; Chauhan, G.; Rath, G. Non-invasive systemic drug delivery through mucosal routes. Artif. Cells Nanomed. Biotechnol. 2018, 46, 539–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, J.C.; Schlachet, I.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Oral Pharmacokinetics of a Chitosan-Based Nano- Drug Delivery System of Interferon Alpha. Polymers 2019, 11, 1862. [Google Scholar] [CrossRef] [Green Version]
- Jøraholmen, M.W.; Basnet, P.; Acharya, G.; Škalko-Basnet, N. PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. 2017, 113, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, C.-S.; Hwang, S.-K.; Gu, M.-J.; Kim, C.-G.; Kim, S.-K.; Ju, D.-B.; Yun, C.-H.; Kim, H.-J. Mucosal Vaccine Delivery Using Mucoadhesive Polymer Particulate Systems. Tissue Eng. Regen. Med. 2021, 18, 693–712. [Google Scholar] [CrossRef]
- Kondiah, P.P.; Tomar, L.K.; Tyagi, C.; Choonara, Y.E.; Modi, G.; du Toit, L.C.; Kumar, P.; Pillay, V. A novel pH-sensitive interferon-β (INF-β) oral delivery system for application in multiple sclerosis. Int. J. Pharm. 2013, 456, 459–472. [Google Scholar] [CrossRef]
- Rose, F.; Wern, J.E.; Gavins, F.; Andersen, P.; Follmann, F.; Foged, C. A strong adjuvant based on glycol-chitosan-coated lipid-polymer hybrid nanoparticles potentiates mucosal immune responses against the recombinant Chlamydia trachomatis fusion antigen CTH522. J. Control. Release 2018, 271, 88–97. [Google Scholar] [CrossRef]
- Sander, C.; Madsen, K.D.; Hyrup, B.; Nielsen, H.M.; Rantanen, J.; Jacobsen, J. Characterization of spray dried bioadhesive metformin microparticles for oromucosal administration. Eur. J. Pharm. Biopharm. 2013, 85, 682–688. [Google Scholar] [CrossRef]
- Klemetsrud, T.; Kjøniksen, A.L.; Hiorth, M.; Jacobsen, J.; Smistad, G. Polymer coated liposomes for use in the oral cavity—A study of the in vitro toxicity, effect on cell permeability and interaction with mucin. J. Liposome Res. 2018, 28, 62–73. [Google Scholar] [CrossRef]
- Pilicheva, B.; Uzunova, Y.; Bodurov, I.; Viraneva, A.; Exner, G.; Sotirov, S.; Yovcheva, T.; Marudova, M. Layer-by-layer self-assembly films for buccal drug delivery: The effect of polymer cross-linking. J. Drug Deliv. Sci. Technol. 2020, 59, 101897. [Google Scholar] [CrossRef]
- Chaves, P.D.S.; Ourique, A.; Frank, L.A.; Pohlmann, A.; Guterres, S.; Beck, R.C.R. Carvedilol-loaded nanocapsules: Mucoadhesive properties and permeability across the sublingual mucosa. Eur. J. Pharm. Biopharm. 2017, 114, 88–95. [Google Scholar] [CrossRef]
- Shahzad, Y.; Maqbool, M.; Hussain, T.; Yousaf, A.M.; Khan, I.U.; Mahmood, T.; Jamshaid, M. Natural and semisynthetic polymers blended orodispersible films of citalopram. Nat. Prod. Res. 2019, 34, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chonkar, A.D.; Rao, J.V.; Managuli, R.S.; Mutalik, S.; Dengale, S.; Jain, P.; Udupa, N. Development of fast dissolving oral films containing lercanidipine HCl nanoparticles in semicrystalline polymeric matrix for enhanced dissolution and ex vivo permeation. Eur. J. Pharm. Biopharm. 2016, 103, 179–191. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Rodrigues, F.S.C.; Campos, A.; Martins, J.; Ambrósio, A.F.; Campos, E.J. Emerging Trends in Nanomedicine for Improving Ocular Drug Delivery: Light-Responsive Nanoparticles, Mesoporous Silica Nanoparticles, and Contact Lenses. ACS Biomater. Sci. Eng. 2020, 6, 6587–6597. [Google Scholar] [CrossRef]
- Awwad, S.; Ahmed, A.M.; Sharma, G.; Heng, J.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef]
- Kopacz, D.; Niezgoda, Ł.; Fudalej, E.; Nowak, A.; Maciejewicz, P. Tear Film–Physiology and Disturbances in Various Diseases and Disorders. In Ocular Surface Diseases: Some Current Date on Tear Film Problem and Keratoconic Diagnosis; IntechOpen: London, UK, 2020. [Google Scholar]
- Vaajanen, A.; Vapaatalo, H. A Single Drop in the Eye—Effects on the Whole Body? Open Ophthalmol. J. 2017, 11, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Srinivasarao, D.A.; Lohiya, G.; Katti, D.S. Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1548. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar]
- Žiniauskaitė, A.; Cėpla, V.; Jelinskas, T.; Eimont, R.; Ulčinas, A.; Aldonytė, R.; Valiokas, R.; Kalesnykas, G.; Hakkarainen, J.J. Introducing an Efficient In Vitro Cornea Mimetic Model for Testing Drug Permeability. Science 2021, 3, 30. [Google Scholar] [CrossRef]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. Eye: Anatomy, physiology and barriers to drug delivery. In Ocular Transporters and Receptors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–36. [Google Scholar]
- Toffoletto, N.; Chauhan, A.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A. Asymmetry in Drug Permeability through the Cornea. Pharmaceutics 2021, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Auriola, S.; Grey, A.; Demarais, N.; Valtari, A.; Heikkinen, E.; Toropainen, E.; Urtti, A.; Vellonen, K.-S.; Ruponen, M. Partitioning and Spatial Distribution of Drugs in Ocular Surface Tissues. Pharmaceutics 2021, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.; Swindle-Reilly, K.E. Rheological Properties and Age-Related Changes of the Human Vitreous Humor. Front. Bioeng. Biotechnol. 2018, 6, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bévalot, F.; Cartiser, N.; Bottinelli, C.; Fanton, L.; Guitton, J. Vitreous humor analysis for the detection of xenobiotics in forensic toxicology: A review. Forensic Toxicol. 2015, 34, 12–40. [Google Scholar] [CrossRef] [Green Version]
- Käsdorf, B.T.; Arends, F.; Lieleg, O. Diffusion Regulation in the Vitreous Humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Serpe, L.; Foglietta, F.; Muntoni, E.; Rodriguez, A.D.P.; Aspiazu, M.S. Ocular delivery of solid lipid nanoparticles. In Lipid Nanocarriers for Drug Targeting; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 269–312. [Google Scholar]
- Tomi, M.; Hosoya, K.-I. The role of blood–ocular barrier transporters in retinal drug disposition: An overview. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.-S.; Rupenthal, I.D. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 2018, 126, 96–112. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2018, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, X.; Chen, X.; Wang, B.; Xu, W. Intellective and stimuli-responsive drug delivery systems in eyes. Int. J. Pharm. 2021, 602, 120591. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Lai, J.-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Aziz, A.B.A.; Bhattamisra, S.; Gorain, B.; Su, J.; Tan, C.; Chin, W.; Yip, K. Potential of Stimuli-Responsive In Situ Gel System for Sustained Ocular Drug Delivery: Recent Progress and Contemporary Research. Polymers 2021, 13, 1340. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, C.; Zhu, Q.; Zhang, X.; Guan, J.; Mao, S. Comparison of thermosensitive in situ gels and drug-resin complex for ocular drug delivery: In vitro drug release and in vivo tissue distribution. Int. J. Pharm. 2020, 578, 119184. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, J.; Li, Y.; Huang, J.; Huang, Z.; Huang, Y.; Pan, X.; Wu, C. Thermo-sensitive gel in glaucoma therapy for enhanced bioavailability: In vitro characterization, in vivo pharmacokinetics and pharmacodynamics study. Life Sci. 2018, 212, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, H.; Gu, D.; Sun, H.; Chen, K.; Tan, G.; Pan, W. A Novel Carbon Dots/Thermo-Sensitive In Situ Gel for a Composite Ocular Drug Delivery System: Characterization, Ex-Vivo Imaging, and In Vivo Evaluation. Int. J. Mol. Sci. 2021, 22, 9934. [Google Scholar] [CrossRef]
- Andrés-Guerrero, V.; Bravo-Osuna, I.; Pastoriza, P.; Molina-Martinez, I.T.; Herrero-Vanrell, R. Novel technologies for the delivery of ocular therapeutics in glaucoma. J. Drug Deliv. Sci. Technol. 2017, 42, 181–192. [Google Scholar] [CrossRef]
- El-Feky, Y.A.; Fares, A.R.; Zayed, G.; El-Telbany, R.F.A.; Ahmed, K.A. Repurposing of nifedipine loaded in situ ophthalmic gel as a novel approach for glaucoma treatment. Biomed. Pharmacother. 2021, 142, 112008. [Google Scholar] [CrossRef]
- Okur, N.; Yozgatlı, V.; Okur, M.E.; Yoltaş, A.; Siafaka, P.I. Improving therapeutic efficacy of voriconazole against fungal keratitis: Thermo-sensitive in situ gels as ophthalmic drug carriers. J. Drug Deliv. Sci. Technol. 2018, 49, 323–333. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Li, N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1282–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Benzoic acid derivative-modified chitosan-g-poly(N-isopropylacrylamide): Methoxylation effects and pharmacological treatments of Glaucoma-related neurodegeneration. J. Control. Release 2019, 317, 246–258. [Google Scholar] [CrossRef]
- Toit, L.; Choonara, Y.; Pillay, V. An Injectable Nano-Enabled Thermogel to Attain Controlled Delivery of p11 Peptide for the Potential Treatment of Ocular Angiogenic Disorders of the Posterior Segment. Pharmaceutics 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-Responsive PLGA-PEG-PLGA Hydrogels as Novel Injectable Platforms for Neuroprotective Combined Therapies in the Treatment of Retinal Degenerative Diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef]
- Lin, D.; Lei, L.; Shi, S.; Li, X. Stimulus-Responsive Hydrogel for Ophthalmic Drug Delivery. Macromol. Biosci. 2019, 19, e1900001. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Basri, S.M.M.; Mirshekari, H.; Amiri, M.; Pishabad, Z.S.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [Green Version]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.T.; Ah-Kee, E.Y.; Collins, C.E. Common eye drops and their implications for pH measurements in the management of chemical eye injuries. Int. J. Ophthalmol. 2014, 7, 1067–1068. [Google Scholar] [CrossRef]
- Alruwaili, N.K.; Zafar, A.; Imam, S.S.; Alharbi, K.S.; Alotaibi, N.H.; Alshehri, S.; Alhakamy, N.A.; Alzarea, A.I.; Afzal, M.; Elmowafy, M. Stimulus Responsive Ocular Gentamycin-Ferrying Chitosan Nanoparticles Hydrogel: Formulation Optimization, Ocular Safety and Antibacterial Assessment. Int. J. Nanomed. 2020, 15, 4717–4737. [Google Scholar] [CrossRef]
- Ni, X.; Guo, Q.; Zou, Y.; Xuan, Y.; Mohammad, I.S.; Ding, Q.; Hu, H. Preparation and characterization of bear bile-loaded pH sensitive in-situ gel eye drops for ocular drug delivery. Iran. J. Basic Med. Sci. 2020, 23, 922–929. [Google Scholar] [CrossRef]
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, S.; Yu, S.; Li, J.; Tan, G.; Li, S.; Pan, W. A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, ex Vivo, and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2020, 6, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, I.M.; Korzhikov-Vlakh, V.A.; Zakharova, N.V.; Urtti, A.; Tennikova, T. BThermo- and pH-sensitive glycosaminoglycans derivatives obtained by controlled grafting of poly(N-isopropylacrylamide). Carbohydr. Polym. 2020, 248, 116764. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Waghule, T.; Rapalli, V.K.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Advanced Hydrogels Based Drug Delivery Systems for Ophthalmic Delivery. Recent Patents Drug Deliv. Formul. 2020, 13, 291–300. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Fernández-Ferreiro, A.; Barcia, M.G.; Gil-Martínez, M.; Vieites-Prado, A.; Lema, I.; Argibay, B.; Méndez, J.B.; Lamas, M.J.; Otero-Espinar, F.J. In vitro and in vivo ocular safety and eye surface permanence determination by direct and Magnetic Resonance Imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. Eur. J. Pharm. Biopharm. 2015, 94, 342–351. [Google Scholar] [CrossRef]
- Bhalerao, H.; Koteshwara, K.B.; Chandran, S. Levofloxacin Hemihydrate In Situ Gelling Ophthalmic Solution: Formulation Optimization and In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2019, 20, 272. [Google Scholar] [CrossRef]
- Sai, N.; Dong, X.; Huang, P.; You, L.; Yang, C.; Liu, Y.; Wang, W.; Wu, H.; Yu, Y.; Du, Y.; et al. A Novel Gel-Forming Solution Based on PEG-DSPE/Solutol HS 15 Mixed Micelles and Gellan Gum for Ophthalmic Delivery of Curcumin. Molecules 2019, 25, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Raghava, S. Smart systems based on polysaccharides. In Natural-Based Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2008; pp. 129–161. [Google Scholar]
- Li, P.; Wang, S.; Chen, H.; Zhang, S.; Yu, S.; Li, Y.; Cui, M.; Pan, W.; Yang, X. A novel ion-activated in situ gelling ophthalmic delivery system based on κ-carrageenan for acyclovir. Drug Dev. Ind. Pharm. 2018, 44, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ferreiro, A.; Silva-Rodríguez, J.; Espinar, F.J.O.; González-Barcia, M.; Lamas, M.J.; Ruibal, A.; Luaces-Rodríguez, A.; Vieites-Prado, A.; Lema, I.; Herranz, M.; et al. In vivo eye surface residence determination by high-resolution scintigraphy of a novel ion-sensitive hydrogel based on gellan gum and kappa-carrageenan. Eur. J. Pharm. Biopharm. 2017, 114, 317–323. [Google Scholar] [CrossRef] [PubMed]



| Formulation | Outcome | Reference |
|---|---|---|
| Polylactic acid-based microneedles loaded with sulforhodamine B | Microneedles provided continuous drug delivery and successful skin recovery without any trace of injury | [64] |
| Poly-vinyl pyrrolidone and PVA microneedles loaded with fluorescein isothiocyanate | Microneedles ensured an effective skin penetration ability and controllable drug release | [66] |
| PVA-based microneedles loaded with doxorubicin | Microneedles enhanced transdermal delivery of doxorubicin | [67] |
| Swelling-modified silk fibroin microneedles loaded with 2-ethoxyethanol | Microneedles were able to penetrate into porcine skin in vitro and form hydrogels | [68] |
| Polymeric System | Formulation | Outcome | Reference |
|---|---|---|---|
| Pre-activated thiolated polymers and in situ gels | Xanthan gum and 2-((2-amino-2-carboxyethyl)disulfanyl)nicotinic acid conjugate | Improved mucoadhesion and stability of liquid formulation compared to either regular xanthan gum or thiolated xanthan gum; no negative effects on ciliary beating | [78] |
| Cationic polymers and in situ gels | Aminated gellan gum | Increased viscosity, adhesion time and bioavailability compared to non-modified gellan gum | [32] |
| Cationic polymers | Acrylated Eudragit® E PO (EPO) loaded with fluorescein | Increased adhesion to and retention on mucosa compared to non-modified polymer | [31] |
| Cationic polymers | Complexes of cationic cyclodextrin-polyethylenimine 2k conjugate (CP 2k) and anionic mRNA encoding HIV gp120 | Prolonged retention on nasal epithelium; enhanced humoral and cellular response compared to free mRNA. | [90] |
| Nanoparticles | Chitosan/cyclodextrin nanoparticles loaded with IFN-β | Improved symptoms in mouse models of autoimmune encephalomyelitis | [89] |
| Strategy of Immobilization | Formulation | Outcome | Reference |
|---|---|---|---|
| (PEG)-modified nanoparticles | IFN-α (PEG)-modified chitosan nanoparticles | Provided detectable levels of IFN-α in plasma within 60 min | [103] |
| Polyelectrolyte microparticles | Polyelectrolyte complex of N-trimethyl chitosan copolymer methacrylic acid PEGDMA loaded with INF-β | Increased INF-β plasma concentrations compared to the subcutaneous injection formulation | [106] |
| Cationic polymers | Spray dried particles of chitosan loaded with metformin | Improved encapsulation efficiency for decreased chitosan/metformin ratio | [108] |
| Liposomes coated with cationic or anionic polymers | Chitosan, low-methoxylated pectin, high-methoxylated pectin, amidated pectin, Eudragit, (p(NIPAAM-co-MAA)), and other polymers | The positively charged DDS exhibited the strongest mucoadhesive interaction | [109] |
| Polyelectrolyte complexes | Polyelectrolyte complexes of chitosan and casein loaded with benzydamine | Improved drug absorption and release | [110] |
| Nanocapsules | Nanocapsules based on poly(e-caprolactone) loaded with Carvedilol (CAR) (CAR-LNC) and Eudragit ÒRS 100 (CAR-NC) | Enhanced drug release from the nanocapsules | [111] |
| Polymeric System | Formulation | Outcome | Reference |
|---|---|---|---|
| Thermosensitive in situ gel with nonionic triblock copolymers | - Poloxamer 407 and poloxamer 188 loaded with timolol maleate, - Poloxamer 407 and poloxamer 188 modified with C-dots for delivery of diclofenac sodium | Increased pre-corneal retention time, bioavailability, steadily decreased intraocular pressure | [140,141] |
| Thermosensitive in situ gel with nonionic triblock copolymer and semi-synthetic cellulose polymer derivatives | - Poloxamer 407 and hydroxypropyl methyl cellulose loaded with nifedipine, - Poloxamer 407 and carboxymethylcellulose loaded with voriconazole | Demonstrated sustained release of the drug, decreased intraocular pressure and provided high loading capacity | [143,144] |
| Thermosensitive in situ gel with pNIPAAM copolymer and natural polymers | - pNIPAAM and hyaluronic acid loaded with ketoconazole, - Chitosan and pNIPAAM modified with benzoic acid derivatives loaded with pilocarpine and RGFP966 | Demonstrated high loading capacity, sustained release, improved neuroprotective properties and antioxidant activities of the drug | [145,146] |
| Thermosensitive in situ gel with PLGA and synthetic copolymers | - PLGA nanoparticles embedded with PEG and Pluronic F 127 loaded with p11 hexapeptide, - PLGA and PEG loaded with dexamethasone, ketorolac and idebenone | Increased antioxidative and anti-inflammatory effects of the drug, showed sustained release of the drug and low polydispersity of the gel | [147,148] |
| pH-sensitive in situ gel with carbopol and natural polymers | - Carbopol 974P and chitosan nanoparticles loaded with gentamycin, - Carbopol 974 and hydroxypropyl methylcellulose loaded with bear bill, - Carbopol 934P and hydroxyethyl cellulose loaded with vancomycin niosomes | Increased retention time and bioavailability, demonstrated high drug content, sustained release and greater effect of the loaded drug | [155,156,157] |
| pH-sensitive and thermosensitive in situ gelling polymers | - Carboxymethyl chitosan and poloxamer 407 cross-linked with a naturally occurring cross-linker genipin for delivery of quercetin, - Heparin and chondroitin sulfate loaded with dexamethasone | Increased swelling ratio, demonstrated more controlled and prolonged release of the drug due to dual sensitivity, increased precorneal retention time with great encapsulation | [158,159] |
| Ion sensitive in situ gelling polymer with gellan gum | - Gellan gum loaded with levofloxacin, - PEG-DSPE/polyoxyethylene esters of 12-hydroxystearic acid (Solutol HS 15) mixed micelle and gellan gum loaded with curcumin, - Gellan gum and natamycin bilosomes loaded with natamycin | Demonstrated fast gelling time, high drug content, enhanced solubility and chemical stability, prolonged precorneal residence and release of the drug, increased corneal permeability and persistence on the ocular surface | [163,164,165] |
| Ion sensitive in situ gel with a natural linear polymeric polysaccharide | - Kappa-carrageenan modified by hydroxypropyl-β-CD and hydroxypropyl methylcellulose for delivery of acyclovir, - Kappa-carrageenan and gellan gum loaded with radiotracers for scintigraphy | Prolonged release of the agent, increased viscosity and absorption of the drug, improved retention time and bioavailability | [167,168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berillo, D.; Zharkinbekov, Z.; Kim, Y.; Raziyeva, K.; Temirkhanova, K.; Saparov, A. Stimuli-Responsive Polymers for Transdermal, Transmucosal and Ocular Drug Delivery. Pharmaceutics 2021, 13, 2050. https://doi.org/10.3390/pharmaceutics13122050
Berillo D, Zharkinbekov Z, Kim Y, Raziyeva K, Temirkhanova K, Saparov A. Stimuli-Responsive Polymers for Transdermal, Transmucosal and Ocular Drug Delivery. Pharmaceutics. 2021; 13(12):2050. https://doi.org/10.3390/pharmaceutics13122050
Chicago/Turabian StyleBerillo, Dmitriy, Zharylkasyn Zharkinbekov, Yevgeniy Kim, Kamila Raziyeva, Kamila Temirkhanova, and Arman Saparov. 2021. "Stimuli-Responsive Polymers for Transdermal, Transmucosal and Ocular Drug Delivery" Pharmaceutics 13, no. 12: 2050. https://doi.org/10.3390/pharmaceutics13122050