Silk Fibroin: An Ancient Material for Repairing the Injured Nervous System
Abstract
:1. Introduction
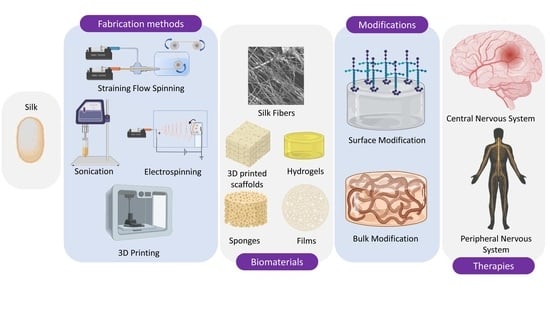
2. The Source of Silk Fibroin: From Nature to Laboratory
- Forcibly spinning of the fibers from the silk-producing species (mainly spiders) [43,44]. Spider silk fibers are biocompatible, after the removal of the possible contaminants, and are commonly used as guiding biomaterial [44]. Spider silk lacks the sericin coating, which is present in the forcibly silked fibers retrieve from silkworms [45]. However, the usage of this method is complex, cost-intensive, and—most importantly—provides only very small quantities (up to mg), far from the required amounts for the biomedical context outside the laboratory.
- Extraction of the proteins from the natural source (mainly from the silkworm). This extraction can be done directly from the silk glands of the animal [46,47] or by dissolving the proteins of the spun fibers during the process of degumming and regeneration [48]. This latter method is the most common for tissue engineering applications and comprises the largest proportion of reported research in neural tissue engineering (Table 1), so that it will be the main focus of this review.
- Production of recombinant silk proteins by cloning the gene expressing the silk fibroin in a host organism [49,50,51]. This approach can be a good answer to provide enough silk biomaterial with tailored properties for clinical usage. Silk genes can be modified to further enhance the quality and tunability of the final biomaterial [51]. However, the recombinant production of silk show limitations due to the different amino acid codon preference of the host organism with respect to the donor insects. This limitation, as well as the possible contamination with pyrogens, becomes more significant in the case of bacterial hosts.
3. Fabrication Methods and Platforms for Regenerated SF-Based Biomaterials
3.1. Fabrication of Non-Fiber-Based SF Biomaterials
3.1.1. Sponges
3.1.2. Silk Hydrogels
3.1.3. Silk Films
3.1.4. Solid Free Form Formats
3.2. Fabrication of Fiber-Based Biomaterials
3.2.1. SF Fibers
3.2.2. Silk Mats
4. Silk Fibroin to Revert Pathological Conditions of Stroke
5. Silk Fibroin in the Context of Neurodegenerative Diseases
6. The Importance of Silk in Peripheral Nerve Injury
7. Increasing the Performance of Silk Fibroin in Neural Tissue Engineering
7.1. Improvement of Cell Adhesion
7.2. Growth Factors and Drug Delivery
7.3. Tuning the Biomechanical Properties and Degradation
7.4. Providing Alignment and Polarity
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortora, G.J.; Derrickson, B.H. Principles of Anatomy and Physiology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- StemCells Inc. A Single-Blind, Randomized, Parallel Arm, Phase II Proof-of-Concept Study of the Safety and Efficacy of Human Central Nervous System Stem Cells (HuCNS-SC) Transplantation in Cervical Spinal Cord Injury; US National Library of Medicine: Bethesda, MD, USA, 2016.
- Lineage Cell Therapeutics Inc. A Phase 1/2a Dose Escalation Study of AST-OPC1 in Subjects with Subacute Cervical Spinal Cord Injury; US National Library of Medicine: Bethesda, MD, USA, 2020.
- Cyto Therapeutics Pty Limited. A Single Arm, Open-Label Phase 1 Study to Evaluate the Safety and Tolerability of ISC-hpNSC Injected into the Striatum and Substantia Nigra of Patients With Parkinson’s Disease; US National Library of Medicine: Bethesda, MD, USA, 2019.
- Pereira, I.M.; Marote, A.; Salgado, A.J.; Silva, N.A. Filling the Gap: Neural Stem Cells as A Promising Therapy for Spinal Cord Injury. Pharmaceuticals 2019, 12, 65. [Google Scholar] [CrossRef] [Green Version]
- Shroff, G.; Gupta, R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Ann. Neurosci. 2015, 22, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.-H.; Fan, H.-C.; Hueng, D.-Y. Potential of Neural Stem Cell-Based Therapy for Parkinson’s Disease. Parkinsons Dis. 2015, 2015, 571475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamali, F. A Safety and Efficacy Study of the Effects of Mesenchymal Stem Cells (MSCs) Differentiated into Neural Stem Cells (NSCs) on the Motor and Non-motor Symptoms in People with Parkinson’s Disease (PD); US National Library of Medicine: Bethesda, MD, USA, 2020.
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem. Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-El-Enein, M.; Elsanhoury, A.; Reinke, P. Overcoming Challenges Facing Advanced Therapies in the EU Market. Cell Stem. Cell 2016, 19, 293–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Munoz, J.R.; Stoutenger, B.R.; Robinson, A.P.; Spees, J.L.; Prockop, D.J. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc. Natl. Acad. Sci. USA 2005, 102, 18171–18176. [Google Scholar] [CrossRef] [Green Version]
- Ohtaki, H.; Ylostalo, J.H.; Foraker, J.E.; Robinson, A.P.; Reger, R.L.; Shioda, S.; Prockop, D.J. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc. Natl. Acad. Sci. USA 2008, 105, 14638. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, Z.; Rege, S.V.; Wang, M.; Si, G.; Zhou, Y.; Wang, S.; Griffin, J.H.; Goldman, S.A.; Zlokovic, B.V. 3K3A–activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat. Med. 2016, 22, 1050–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.J.; Elisseeff, J.H. Mimicking biological functionality with polymers for biomedical applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef]
- Cho, D.-W.; Lee, J.-S.; Jang, J.; Jung, J.W.; Park, J.H.; Pati, F. Natural, synthetic and semi-synthetic polymers. In Organ Printing; Morgan & Claypool Publishers: San Rafael, CA, USA, 2015; pp. 7-1–7-10. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Schutgens, E.M.; Tryfonidou, M.A.; Smit, T.H.; Oner, F.C.; Krouwels, A.; Ito, K.; Creemers, L.B. Biomaterials for intervertebral disc regeneration: Past performance and possible future strategies. Eur. Cell Mater. 2015, 30, 210–231. [Google Scholar] [CrossRef] [Green Version]
- Faulk, D.M.; Badylak, S.F. Chapter 8—Natural Biomaterials for Regenerative Medicine Applications. In Regenerative Medicine Applications in Organ Transplantation; Orlando, G., Lerut, J., Soker, S., Stratta, R.J., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 101–112. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Jiang, Z.; Li, N.; Liu, P.; Liu, L.; Tang, M.; Cheng, G. Anti-inflammatory effects of three-dimensional graphene foams cultured with microglial cells. Biomaterials 2014, 35, 6930–6940. [Google Scholar] [CrossRef] [PubMed]
- Rosso, F.; Marino, G.; Giordano, A.; Barbarisi, M.; Parmeggiani, D.; Barbarisi, A. Smart materials as scaffolds for tissue engineering. J. Cell. Physiol. 2005, 203, 465–470. [Google Scholar] [CrossRef]
- Maughan, E.F.; Hynds, R.E.; Proctor, T.J.; Janes, S.M.; Elliott, M.; Birchall, M.A.; Lowdell, M.W.; De Coppi, P. Autologous Cell Seeding in Tracheal Tissue Engineering. Curr. Stem. Cell Rep. 2017, 3, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F. Biocompatibility pathways: Biomaterials-induced sterile inflammation, mechanotransduction, and principles of biocompatibility control. ACS Biomater. Sci. Eng. 2017, 3, 2–35. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R. Smart biomaterials: Recent advances and future directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Ravichandran, R.; Astrand, C.; Patra, H.K.; Turner, A.P.; Chotteau, V.; Phopase, J. Intelligent ECM mimetic injectable scaffolds based on functional collagen building blocks for tissue engineering and biomedical applications. RSC Adv. 2017, 7, 21068–21078. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobin, A.S.; Rhea, R.; Newman, R.A.; Mathur, A.B. Silk-fibroin-coated liposomes for long-term and targeted drug delivery. Int. J. Nanomed. 2006, 1, 81. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood–brain barrier. Int. J. Nanomed. 2016, 11, 5381. [Google Scholar] [CrossRef] [Green Version]
- Tang-Schomer, M.D.; Kaplan, D.L.; Whalen, M.J. Film interface for drug testing for delivery to cells in culture and in the brain. Acta Biomater. 2019, 94, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, L.; Gong, D.; Yin, H.; Zhang, J. Biomolecular evidence of silk from 8,500 years ago. PLoS ONE 2016, 11, e0168042. [Google Scholar]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Theodora, C.; Sara, P.; Silvio, F.; Alessandra, B.; Giuseppe, T.; Barbara, V.; Barbara, C.; Sabrina, R.; Silvia, D.; Stefania, P.; et al. Platelet lysate and adipose mesenchymal stromal cells on silk fibroin nonwoven mats for wound healing. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Bray, L.J.; George, K.A.; Hutmacher, D.W.; Chirila, T.V.; Harkin, D.G. A dual-layer silk fibroin scaffold for reconstructing the human corneal limbus. Biomaterials 2012, 33, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q. SDS-PAGE for silk fibroin protein. Bio Protocol. 2018, e3054. [Google Scholar] [CrossRef]
- Mu, X.; Fitzpatrick, V.; Kaplan, D.L. From silk spinning to 3D printing: Polymer manufacturing using directed hierarchical molecular assembly. Adv. Funct. Mater. 2020, 9, 1901552. [Google Scholar] [CrossRef]
- Work, R.W.; Emerson, P.D. An Apparatus and Technique for the Forcible Silking of Spiders. J. Arachnol. 1982, 10, 1–10. [Google Scholar]
- Roloff, F.; Strauß, S.; Vogt, P.M.; Bicker, G.; Radtke, C. Spider silk as guiding biomaterial for human model neurons. BioMed Res. Int. 2014, 2014, 906819. [Google Scholar] [CrossRef]
- Römer, L.; Scheibel, T. The elaborate structure of spider silk: Structure and function of a natural high performance fiber. Prion 2008, 2, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-K.; Oh, H.-S.; Cho, Y.-H.; Kim, Y.-J.; Han, Y.-G.; Nam, S.-H. Effects of a silkworm extract on dopamine and monoamine oxidase-B activity in an MPTP-induced Parkinsons disease model. Lab. Anim. Res. 2010, 26, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-K.; Nam, S.-H.; Sohn, H.-O.; Lee, D.-W. Inhibitory Effect of Silkworm-Extract(SE) on Monoamine Oxidase Activity in Vitro and in Vivo. Entomol. Res. 2005, 35, 189–193. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2012, 30, 217–224. [Google Scholar] [CrossRef]
- Gonska, N.; López, P.A.; Lozano-Picazo, P.; Thorpe, M.; Guinea, G.V.; Johansson, J.; Barth, A.; Pérez-Rigueiro, J.; Rising, A. Structure–Function Relationship of Artificial Spider Silk Fibers Produced by Straining Flow Spinning. Biomacromolecules 2020, 21, 2116–2124. [Google Scholar] [CrossRef]
- Heidebrecht, A.; Eisoldt, L.; Diehl, J.; Schmidt, A.; Geffers, M.; Lang, G.; Scheibel, T. Biomimetic Fibers Made of Recombinant Spidroins with the Same Toughness as Natural Spider Silk. Adv. Mater. 2015, 27, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.B.; DeSimone, E.; Scheibel, T. Biomedical applications of recombinant silk-based materials. Adv. Mater. 2018, 30, 1704636. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rigueiro, J.; Madurga, R.; Gañán-Calvo, A.M.; Plaza, G.R.; Elices, M.; López, P.A.; Daza, R.; González-Nieto, D.; Guinea, G.V. Straining flow spinning of artificial silk fibers: A review. Biomimetics 2018, 3, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercado, J.; Pérez-Rigueiro, J.; González-Nieto, D.; Lozano-Picazo, P.; López, P.; Panetsos, F.; Elices, M.; Gañán-Calvo, A.M.; Guinea, G.V.; Ramos-Gómez, M. Regenerated Silk Fibers Obtained by Straining Flow Spinning for Guiding Axonal Elongation in Primary Cortical Neurons. ACS Biomater. Sci. Eng. 2020, 6, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Reagan, M.R.; Kaplan, D.L. Electrospun silk biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 988–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Liu, R.; Zuo, B.Q.; Qin, J.Z. Electrospun silk fibroin nanofiber tubes for peripheral nerve regeneration. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Qu, J.; Wang, D.; Wang, H.; Dong, Y.; Zhang, F.; Zuo, B.; Zhang, H. Electrospun silk fibroin nanofibers in different diameters support neurite outgrowth and promote astrocyte migration. J. Biomed. Mater. Res. Part. A 2013, 101, 2667–2678. [Google Scholar] [CrossRef]
- Jiang, J.-P.; Liu, X.-Y.; Zhao, F.; Zhu, X.; Li, X.-Y.; Niu, X.-G.; Yao, Z.-T.; Dai, C.; Xu, H.-Y.; Ma, K. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen. Res. 2020, 15, 959. [Google Scholar]
- Zhao, Y.-H.; Niu, C.-M.; Shi, J.-Q.; Wang, Y.-Y.; Yang, Y.-M.; Wang, H.-B. Novel conductive polypyrrole/silk fibroin scaffold for neural tissue repair. Neural Regen. Res. 2018, 13, 1455. [Google Scholar]
- Chwalek, K.; Sood, D.; Cantley, W.L.; White, J.D.; Tang-Schomer, M.; Kaplan, D.L. Engineered 3D silk-collagen-based model of polarized neural tissue. Jove J. Vis. Exp. 2015, e52970. [Google Scholar] [CrossRef] [Green Version]
- Chwalek, K.; Tang-Schomer, M.D.; Omenetto, F.G.; Kaplan, D.L. In vitro bioengineered model of cortical brain tissue. Nat. Protoc 2015, 10, 1362–1373. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wray, L.S.; Burke, K.A.; Torregrosa, T.; Golinski, J.M.; Huang, W.; Kaplan, D.L. Lyophilized Silk Sponges: A Versatile Biomaterial Platform for Soft Tissue Engineering. ACS Biomater. Sci. Eng. 2015, 1, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-García, L.; Pérez-Rigueiro, J.; Martinez-Murillo, R.; Panetsos, F.; Ramos, M.; Guinea, G.V.; González-Nieto, D. Cortical reshaping and functional recovery induced by silk fibroin hydrogels-encapsulated stem cells implanted in stroke animals. Front. Cell. Neurosci. 2018, 12, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Serra, R.; Gallego, R.; Lozano, P.; González-Nieto, D. Hydrogels for neuroprotection and functional rewiring: A new era for brain engineering. Neural Regen. Res. 2020, 15, 783. [Google Scholar] [PubMed]
- Hronik-Tupaj, M.; Raja, W.K.; Tang-Schomer, M.; Omenetto, F.G.; Kaplan, D.L. Neural responses to electrical stimulation on patterned silk films. J. Biomed. Mater. Res. Part. A 2013, 101, 2559–2572. [Google Scholar] [CrossRef] [Green Version]
- Tang-Schomer, M.D.; Hu, X.; Hronik-Tupaj, M.; Tien, L.W.; Whalen, M.J.; Omenetto, F.G.; Kaplan, D.L. Film-based implants for supporting neuron–electrode integrated interfaces for the brain. Adv. Funct. Mater. 2014, 24, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Johansson, J.; Rising, A. Silk spinning in silkworms and spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.G.; Scheibel, T.R. Production and processing of spider silk proteins. J. Polym. Sci. Part. A Polym. Chem. 2009, 47, 3957–3963. [Google Scholar] [CrossRef] [Green Version]
- Landry, M.J.; Rollet, F.d.r.-G.; Kennedy, T.E.; Barrett, C.J. Layers and multilayers of self-assembled polymers: Tunable engineered extracellular matrix coatings for neural cell growth. Langmuir 2018, 34, 8709–8730. [Google Scholar] [CrossRef]
- Kwon, K.-J.; Seok, H. Silk Protein-Based Membrane for Guided Bone Regeneration. Appl. Sci. 2018, 8, 1214. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.-J.; Li, Y.; Yao, S.-W.; He, J.-H. Silkworm-based silk fibers by electrospinning. Results Phys. 2019, 15, 102646. [Google Scholar] [CrossRef]
- Lotz, B.; Brack, A.; Spach, G. β Structure of periodic copolypeptides of l-alanine and glycine: Their relevance to the structure of silks. J. Mol. Biol. 1974, 87, 193–203. [Google Scholar] [CrossRef]
- Shimizu, M. Eine röntgenographische Untersuchung des Sericins. Bull. Sericult. Exp. Stn 1941, 10, 441–474. [Google Scholar]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Chankow, S.; Luemunkong, S.; Kanokpanont, S. Conformational transitions of thai silk fibroin secondary structures. In Proceedings of the 2016 9th Biomedical Engineering International Conference (BMEiCON), Laung Prabang, Laos, 7–9 December 2016; pp. 1–5. [Google Scholar]
- Yi, B.; Zhang, H.; Yu, Z.; Yuan, H.; Wang, X.; Zhang, Y. Fabrication of high performance silk fibroin fibers via stable jet electrospinning for potential use in anisotropic tissue regeneration. J. Mater. Chem. B 2018, 6, 3934–3945. [Google Scholar] [CrossRef]
- Fernández-García, L.; Marí-Buyé, N.; Barios, J.A.; Madurga, R.; Elices, M.; Pérez-Rigueiro, J.; Ramos, M.; Guinea, G.V.; González-Nieto, D. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. Acta Biomater. 2016, 45, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; De Laporte, L.; Tortelli, F.; Spedden, E.; Staii, C.; Atherton, T.J.; Hubbell, J.A.; Kaplan, D.L. Silk Hydrogels as Soft Substrates for Neural Tissue Engineering. Adv. Funct. Mater. 2013, 23, 5140–5149. [Google Scholar] [CrossRef]
- Sultan, M.T.; Choi, B.Y.; Ajiteru, O.; Hong, D.K.; Lee, S.M.; Kim, H.J.; Ryu, J.S.; Lee, J.S.; Hong, H.; Lee, Y.J.; et al. Reinforced-hydrogel encapsulated hMSCs towards brain injury treatment by trans-septal approach. Biomaterials 2021, 266, 120413. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhang, Q.; Ye, D.; Li, M.; You, R.; Xu, W. Fabrication of porous silk fibroin/cellulose nanofibril sponges with hierarchical structure using a lithium bromide solvent system. Cellulose 2018, 26, 1013–1023. [Google Scholar] [CrossRef]
- Yucel, T.; Cebe, P.; Kaplan, D.L. Vortex-induced injectable silk fibroin hydrogels. Biophys. J. 2009, 97, 2044–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leisk, G.G.; Lo, T.J.; Yucel, T.; Lu, Q.; Kaplan, D.L. Electrogelation for protein adhesives. Adv. Mater. 2010, 22, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, W.; Lu, Q.; Ma, Q.; Kaplan, D.L.; Zhu, H. Silk Nanofiber Hydrogels with Tunable Modulus to Regulate Nerve Stem Cell Fate. J. Mater. Chem B 2014, 2, 6590–6600. [Google Scholar] [CrossRef]
- Li, M.; Li, J. 12—Biodegradation behavior of silk biomaterials. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Kundu, S.C., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 330–348. [Google Scholar]
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation of Bombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2004, 91, 2383–2390. [Google Scholar] [CrossRef]
- Merceron, T.K.; Murphy, S.V. Hydrogels for 3D bioprinting applications. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 249–270. [Google Scholar]
- Ramião, N.G.; Martins, P.S.; Barroso, M.L.; Santos, D.C.; Fernandes, A.A. In vitro degradation of polydimethylsiloxanes in breast implant applications. J. Appl. Biomater. Funct. Mater. 2017, 15, e369–e375. [Google Scholar] [CrossRef] [PubMed]
- Osama, I.; Gorenkova, N.; McKittrick, C.M.; Wongpinyochit, T.; Goudie, A.; Seib, F.P.; Carswell, H.V.O. In vitro studies on space-conforming self-assembling silk hydrogels as a mesenchymal stem cell-support matrix suitable for minimally invasive brain application. Sci. Rep. 2018, 8, 13655. [Google Scholar] [CrossRef]
- Gorenkova, N.; Osama, I.; Seib, F.P.; Carswell, H.V. In vivo evaluation of engineered self-assembling silk fibroin hydrogels after intracerebral injection in a rat stroke model. ACS Biomater. Sci. Eng. 2018, 5, 859–869. [Google Scholar] [CrossRef]
- Sun, W.; Incitti, T.; Migliaresi, C.; Quattrone, A.; Casarosa, S.; Motta, A. Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide. J. Tissue Eng. Regen Med. 2017, 11, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Martín-Martín, Y.; Fernández-García, L.; Sanchez-Rebato, M.H.; Marí-Buyé, N.; Rojo, F.J.; Pérez-Rigueiro, J.; Ramos, M.; Guinea, G.V.; Panetsos, F.; González-Nieto, D. Evaluation of Neurosecretome from Mesenchymal Stem Cells Encapsulated in Silk Fibroin Hydrogels. Sci. Rep. 2019, 9, 8801. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakakis, N. Fabrication of Biocompatible Electro-Conductive Silk Films with Natural Compounds for Tissue Engineering Applications. Ph.D.Thesis, Tufts University, Medford, MA, USA, February 2015. [Google Scholar]
- Manchineella, S.; Thrivikraman, G.; Basu, B.; Govindaraju, T. Surface-functionalized silk fibroin films as a platform to guide neuron-like differentiation of human mesenchymal stem cells. ACS Appl. Mater. Interfaces 2016, 8, 22849–22859. [Google Scholar] [CrossRef]
- Zhu, W.; Castro, N.; Zhang, L.G. Nanotechnology and 3D bioprinting for neural tissue regeneration. In 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 307–331. [Google Scholar]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio. 2020, 6, 100043. [Google Scholar] [CrossRef]
- Chawla, S.; Midha, S.; Sharma, A.; Ghosh, S. Silk-based bioinks for 3D bioprinting. Adv. Healthc. Mater. 2018, 7, 1701204. [Google Scholar] [CrossRef]
- Agostinacchio, F.; Mu, X.; Dire, S.; Motta, A.; Kaplan, D.L. In Situ 3D Printing: Opportunities with Silk Inks. Trends Biotechnol 2020. [Google Scholar] [CrossRef]
- Wang, Q.; Han, G.; Yan, S.; Zhang, Q. 3D Printing of Silk Fibroin for Biomedical Applications. Materials 2019, 12, 504. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Ai, J.; Biazar, E.; Ebrahimi-Barough, S.; Khojasteh, A.; Yazdankhah, M.; Sharifi, S.; Ai, A.; Heidari-Keshel, S. In vivo assessment of a nanofibrous silk tube as nerve guide for sciatic nerve regeneration. Artif. Cells Nanomed. Biotechnol. 2018, 46, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, Q.; Luo, Z.; Yan, S.; You, R. Biofunctionalized silk fibroin nanofibers for directional and long neurite outgrowth. Biointerphases 2019, 14, 061001. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jin, H.-J.; Kaplan, D.L.; Rutledge, G.C. Mechanical Properties of Electrospun Silk Fibers. Macromolecules 2004, 37, 6856–6864. [Google Scholar] [CrossRef]
- Wang, L.; Song, D.; Zhang, X.; Ding, Z.; Kong, X.; Lu, Q.; Kaplan, D.L. Silk–graphene hybrid hydrogels with multiple cues to induce nerve cell behavior. ACS Biomater. Sci. Eng. 2018, 5, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Fryczkowski, R.; Gorczowska, M.; Fryczkowska, B.; Janicki, J. The effect of solvent on the properties of nanofibres obtained by electrospinning from a mixture of poly(3-hydroxybutyrate) and polyaniline. Synth. Met. 2013, 166, 14–21. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ai, J.; Biazar, E.; Faridi-Majidi, R.; Hajati, J.; Ebrahimi-Barough, S.; Heidari, K.S. Investigation of properties of chemically cross-linked silk nanofibrous mat as a nerve guide. Mater. Technol. 2017, 32, 551–559. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- Dinis, T.M.; Vidal, G.; Jose, R.R.; Vigneron, P.; Bresson, D.; Fitzpatrick, V.; Marin, F.; Kaplan, D.L.; Egles, C. Complementary effects of two growth factors in multifunctionalized silk nanofibers for nerve reconstruction. PLoS ONE 2014, 9, e109770. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikbaev, A.; Frischknecht, R.; Heine, M. Brain extracellular matrix retains connectivity in neuronal networks. Sci. Rep. 2015, 5, 14527. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, F.; Yang, Y.; Hu, N.; Wu, H.; Gu, X. Evaluation on in vitro biocompatibility of silk fibroin-based biomaterials with primarily cultured hippocampal neurons. J. Biomed. Mater. Res. Part. A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 91, 166–174. [Google Scholar]
- Moisenovich, M.M.; Plotnikov, E.Y.; Moysenovich, A.M.; Silachev, D.N.; Danilina, T.I.; Savchenko, E.S.; Bobrova, M.M.; Safonova, L.A.; Tatarskiy, V.V.; Kotliarova, M.S.; et al. Effect of Silk Fibroin on Neuroregeneration After Traumatic Brain Injury. Neurochem. Res. 2019, 44, 2261–2272. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.H.; Sung, J.J.; Kim, E.T.; Cho, H.J.; Kim, K.H.; Kang, Y.K.; Kim, S.S.; Kwon, O.S.; Lee, W.B. The effect of BF-7 on the ischemia-induced learning and memory deficits. Korean J. Anat 2005, 38, 181. [Google Scholar]
- Borlongan, C.V. Age of PISCES: Stem-cell clinical trials in stroke. Lancet 2016, 388, 736–738. [Google Scholar] [CrossRef]
- Borlongan, C.V. Preliminary reports of stereotaxic stem cell transplants in chronic stroke patients. Mol. Ther. 2016, 24, 1710–1711. [Google Scholar] [CrossRef] [Green Version]
- González-Nieto, D.; Fernández-García, L.; Pérez-Rigueiro, J.; Guinea, G.V.; Panetsos, F. Hydrogels-assisted cell engraftment for repairing the stroke-damaged brain: Chimera or reality. Polymers 2018, 10, 184. [Google Scholar] [CrossRef] [Green Version]
- Kondziolka, D.; Steinberg, G.K.; Wechsler, L.; Meltzer, C.C.; Elder, E.; Gebel, J.; DeCesare, S.; Jovin, T.; Zafonte, R.; Lebowitz, J. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J. Neurosurg. 2005, 103, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Catanese, L.; Tarsia, J.; Fisher, M. Acute ischemic stroke therapy overview. Circ. Res. 2017, 120, 541–558. [Google Scholar] [CrossRef]
- Callixte, K.-T.; Clet, T.B.; Jacques, D.; Faustin, Y.; François, D.J.; Maturin, T.-T. The pattern of neurological diseases in elderly people in outpatient consultations in Sub-Saharan Africa. BMC Res. Notes 2015, 8, 159. [Google Scholar] [CrossRef] [Green Version]
- cdc. Leading Causes of Death. Available online: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm (accessed on 21 August 2020).
- Zott, B.; Busche, M.A.; Sperling, R.A.; Konnerth, A. What happens with the circuit in Alzheimer’s disease in mice and humans? Annu. Rev. Neurosci. 2018, 41, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Chon, J.-W.; Kim, H.; Jeon, H.-N.; Park, K.; Lee, K.-G.; Yeo, J.-H.; Kweon, H.; Lee, H.-S.; Jo, Y.-Y.; Park, Y.K. Silk fibroin hydrolysate inhibits osteoclastogenesis and induces apoptosis of osteoclasts derived from RAW 264.7 cells. Int. J. Mol. Med. 2012, 30, 1203–1210. [Google Scholar] [CrossRef]
- Do, S.-G.; Park, J.-H.; Nam, H.; Kim, J.-B.; Lee, J.-Y.; Oh, Y.-S.; Suh, J.-G. Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic β cell mass in C57BL/KsJ-db/db mice. J. Vet. Sci. 2012, 13, 339. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.S.; Kang, Y.K.; Shin, Y.K.; Lee, H.J.; Yu, J.I.; Lee, K.G.; Yeo, J.H.; Kim, Y.S.; Sohn, D.S.; Kim, K.Y. The role of BF-7 on neuroprotection and enhancement of cognitive function. Korean J. Physiol. Pharmacol. 2004, 8, 173–180. [Google Scholar]
- Chei, S.; Oh, H.-J.; Lee, K.; Jin, H.; Lee, J.-Y.; Lee, B.-Y. Dietary Silk Peptide Inhibits LPS-Induced Inflammatory Responses by Modulating Toll-Like Receptor 4 (TLR4) Signaling. Biomolecules 2020, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Yellamma, K. Silk protein, sericin as a cognitive enhancer in Alzheimer’s disease. J. Alzheimers Dis. Parkinsonism 2014, 4, 2161-0460. [Google Scholar]
- Kim, D.K.; Kang, Y.K.; Lee, M.Y.; Lee, K.-G.; Yeo, J.-H.; Lee, W.B.; Kim, Y.S.; Kim, S.S. Neuroprotection and enhancement of learning and memory by BF-7. J. Health Sci. 2005, 51, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.K.; Lee, B.Y.; Bucci, L.R.; Stohs, S.J. Effect of a Fibroin Enzymatic Hydrolysate on Memory Improvement: A Placebo-Controlled, Double-Blind Study. Nutrients 2018, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Cha, Y.; Lee, S.H.; Jang, S.K.; Guo, H.; Ban, Y.-H.; Park, D.; Jang, G.Y.; Yeon, S.; Lee, J.-Y.; Choi, E.-K.; et al. A silk peptide fraction restores cognitive function in AF64A-induced Alzheimer disease model rats by increasing expression of choline acetyltransferase gene. Toxicol. Appl. Pharmacol. 2017, 314, 48–54. [Google Scholar] [CrossRef]
- Peera, K.; Yellamma, K. Evaluation of potential antioxidant activity of silk protein–sericin against Alzheimer’s disease induced rat brain. Sci. Spectr. 2016, 1, 384–395. [Google Scholar]
- Peera, K.; Yellamma, K. Sericin as a chlinergic modulator in alzaeimer’s disease induced rat. Int J. Pharm Pharm Sci 2015, 7, 108–112. [Google Scholar]
- Kim, T.K.; Park, D.; Yeon, S.; Lee, S.H.; Choi, Y.J.; Bae, D.-K.; Yang, Y.-H.; Yang, G.; Joo, S.S.; Lim, W.-T. Tyrosine-fortified silk amino acids improve physical function of Parkinson’s disease rats. Food Sci. Biotechnol. 2011, 20, 79–84. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Nicoletti, A.; Mostile, G.; Zappia, M. The Parkinsonian personality: More than just a “trait”. Front. Neurol. 2019, 9, 1191. [Google Scholar] [CrossRef]
- Maharaj, H.; Sukhdev Maharaj, D.; Scheepers, M.; Mokokong, R.; Daya, S. l-DOPA administration enhances 6-hydroxydopamine generation. Brain Res. 2005, 1063, 180–186. [Google Scholar] [CrossRef]
- Hernandez-Baltazar, D.; Zavala-Flores, L.M.; Villanueva-Olivo, A. The 6-hydroxydopamine model and parkinsonian pathophysiology: Novel findings in an older model. Neurologia 2017, 32, 533–539. [Google Scholar] [CrossRef]
- Stansley, B.J.; Yamamoto, B.K. L-dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology 2013, 67, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T.; Treis, A.; Patenge, N.; Fiesel, F.C.; Springer, W.; Kahle, P. Parkin protects against tyrosinase-mediated dopamine neurotoxicity by suppressing stress-activated protein kinase pathways. J. Neurochem. 2008, 105, 1700–1715. [Google Scholar] [CrossRef]
- Banagozar Mohammadi, A.; Sadigh-Eteghad, S.; Torbati, M.; Bagher Fazljou, M.S.; Vatandoust, M.S.; Ej Golzari, S.; Farajdokht, F.; Mahmoudi, J. Identification and applications of neuroactive silk proteins: A narrative review. J. Appl. Biomed. 2019, 17, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Damrongsakkul, S.; Kanokpanont, S.; Srichana, T. Properties and antityrosinase activity of sericin from various extraction methods. Biotechnol. Appl. Biochem. 2010, 55, 91–98. [Google Scholar] [CrossRef]
- DiFrancisco-Donoghue, J.; Rabin, E.; Lamberg, E.M.; Werner, W.G. Effects of Tyrosine on Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Mov. Disord. Clin. Pr. 2014, 1, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, M.; Scarian, E.; Rey, F.; Gagliardi, S.; Carelli, S.; Pansarasa, O.; Cereda, C. Biomaterials in Neurodegenerative Disorders: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2020, 21, 3243. [Google Scholar] [CrossRef]
- Xiong, M.; Tao, Y.; Gao, Q.; Feng, B.; Yan, W.; Zhou, Y.; Kotsonis, T.A.; Yuan, T.; You, Z.; Wu, Z. Human stem cell-derived neurons repair circuits and restore neural function. Cell Stem. Cell 2020, 28, 112–126. [Google Scholar] [CrossRef]
- Sunderland, S.; Smith, J.W. Nerves and nerve injuries. Plast. Reconstr. Surg. 1969, 44, 601. [Google Scholar] [CrossRef]
- Sunderland, S. A classification of peripheral nerve injuries producing loss of function. Brain 1951, 74, 491–516. [Google Scholar] [CrossRef]
- Seddon, H.J. Classification of Nerve Injuries. Br. Med. J. 1942, 2, 438. [Google Scholar] [CrossRef]
- Li, R.; Li, D.-h.; Zhang, H.-y.; Wang, J.; Li, X.-k.; Xiao, J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300. [Google Scholar] [CrossRef]
- Park, Y.H.; Ki, C.S.; Kim, H.J.; Park, S.Y. Silk nanofiber nerve conduit and method for producing thereof. International Application No. PCT/KR2010/005280,, 17 February 2011. [Google Scholar]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [Green Version]
- Nassimizadeh, M.; Nassimizadeh, A.K.; Power, D. Research. Managing the nerve gap: New tools in the peripheral nerve repair toolbox. J. Musculoskelet. Surg. 2019, 3, 4. [Google Scholar] [CrossRef]
- Guedan-Duran, A.; Jemni-Damer, N.; Orueta-Zenarruzabeitia, I.; Guinea, G.V.; Perez-Rigueiro, J.; Gonzalez-Nieto, D.; Panetsos, F. Biomimetic Approaches for Separated Regeneration of Sensory and Motor Fibers in Amputee People: Necessary Conditions for Functional Integration of Sensory–Motor Prostheses With the Peripheral Nerves. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Bojnordi, M.N.; Ebrahimi-Barough, S.; Vojoudi, E.; Hamidabadi, H.G. Silk nanofibrous electrospun scaffold enhances differentiation of embryonic stem like cells derived from testis in to mature neuron. J. Biomed. Mater. Res. Part. A 2018, 106, 2662–2669. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhu, J.; Xue, C.; Li, Z.; Ding, F.; Yang, Y.; Gu, X. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 2014, 35, 2253–2263. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Z.; Huang, J.; Wang, H.; Gu, X.; Gu, J. Application of marrow mesenchymal stem cell-derived extracellular matrix in peripheral nerve tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2250–2260. [Google Scholar] [CrossRef]
- Xue, C.; Zhu, H.; Tan, D.; Ren, H.; Gu, X.; Zhao, Y.; Zhang, P.; Sun, Z.; Yang, Y.; Gu, J. Electrospun silk fibroin-based neural scaffold for bridging a long sciatic nerve gap in dogs. J. Tissue Eng. Regen. Med. 2018, 12, e1143–e1153. [Google Scholar] [CrossRef]
- Cai, K.; Yao, K.; Cui, Y.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Gao, L. Influence of different surface modification treatments on poly (D, L-lactic acid) with silk fibroin and their effects on the culture of osteoblast in vitro. Biomaterials 2002, 23, 1603–1611. [Google Scholar] [CrossRef]
- Chiarini, A.; Petrini, P.; Bozzini, S.; Pra, I.D.; Armato, U. Silk fibroin/poly(carbonate)-urethane as a substrate for cell growth: In vitro interactions with human cells. Biomaterials 2003, 24, 789–799. [Google Scholar] [CrossRef]
- Xu, W.; Yagoshi, K.; Asakura, T.; Sasaki, M.; Niidome, T. Silk Fibroin as a Coating Polymer for Sirolimus-Eluting Magnesium Alloy Stents. Acs Appl. Bio Mater. 2019, 3, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Farrukh, A.; Zhao, S.; Del Campo, A. Microenvironments designed to support growth and function of neuronal cells. Front. Mater. 2018, 5, 62. [Google Scholar] [CrossRef]
- Park, M.; Shin, M.; Kim, E.; Lee, S.; Park, K.I.; Lee, H.; Jang, J.-H. The promotion of human neural stem cells adhesion using bioinspired poly (norepinephrine) nanoscale coating. J. Nanomater. 2014, 2014, 793052. [Google Scholar] [CrossRef]
- Neal, R.A.; Tholpady, S.S.; Foley, P.L.; Swami, N.; Ogle, R.C.; Botchwey, E.A. Alignment and composition of laminin–polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. Part. A 2012, 100, 406–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Chen, K.; You, D.; Xia, M.; Li, W.; Fan, S.; Li, H.; Zhang, Y.; Chai, R.; Sun, S. Laminin-coated electrospun regenerated silk fibroin mats promote neural progenitor cell proliferation, differentiation, and survival in vitro. Front. Bioeng. Biotechnol. 2019, 7, 190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yan, S.; You, R.; Kaplan, D.L.; Liu, Y.; Qu, J.; Li, X.; Li, M.; Wang, X. Multichannel silk protein/laminin grafts for spinal cord injury repair. J. Biomed. Mater. Res. Part. A 2016, 104, 3045–3057. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, Y.; Xu, J.; Song, G.; Ding, M.; Zhao, H.; Wang, J. An RGD-Containing Peptide Derived from Wild Silkworm Silk Fibroin Promotes Cell Adhesion and Spreading. Polymers 2018, 10, 1193. [Google Scholar] [CrossRef] [Green Version]
- Grosheva, M.; Nohroudi, K.; Schwarz, A.; Rink, S.; Bendella, H.; Sarikcioglu, L.; Klimaschewski, L.; Gordon, T.; Angelov, D. Comparison of trophic factors’ expression between paralyzed and recovering muscles after facial nerve injury. A quantitative analysis in time course. Exp. Neurol. 2016, 279, 137–148. [Google Scholar] [CrossRef]
- Lang, E.M.; Schlegel, N.; Reiners, K.; Hofmann, G.O.; Sendtner, M.; Asan, E. Single-dose application of CNTF and BDNF improves remyelination of regenerating nerve fibers after C7 ventral root avulsion and replantation. J. Neurotrauma 2008, 25, 384–400. [Google Scholar] [CrossRef]
- Thoenen, H.; Barde, Y.; Edgar, D. The role of nerve growth factor (NGF) and related factors for the survival of peripheral neurons. Adv. Biochem. Psychopharmacol. 1981, 28, 263–273. [Google Scholar]
- Zhang, Y.; Huang, J.; Huang, L.; Liu, Q.; Shao, H.; Hu, X.; Song, L. Silk fibroin-based scaffolds with controlled delivery order of VEGF and BDNF for cavernous nerve regeneration. ACS Biomater. Sci. Eng. 2016, 2, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Prabhakaran, M.P.; Hu, J.; Chen, M.; Besenbacher, F.; Ramakrishna, S. Coaxial electrospun poly (lactic acid)/silk fibroin nanofibers incorporated with nerve growth factor support the differentiation of neuronal stem cells. Rsc Adv. 2015, 5, 49838–49848. [Google Scholar] [CrossRef]
- Chen, C.S.; Soni, S.; Le, C.; Biasca, M.; Farr, E.; Chen, E.Y.; Chin, W.C. Human stem cell neuronal differentiation on silk-carbon nanotube composite. Nanoscale Res. Lett 2012, 7, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Sharma, M.; Saharia, D.; Sarma, K.K.; Sarma, M.G.; Borthakur, B.B.; Bora, U. In vivo studies of silk based gold nano-composite conduits for functional peripheral nerve regeneration. Biomaterials 2015, 62, 66–75. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Niu, C.; Wei, Z.; Shi, J.; Li, G.; Yang, Y.; Wang, H. A new electrospun graphene-silk fibroin composite scaffolds for guiding Schwann cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 2171–2185. [Google Scholar] [CrossRef]
- Pillai, M.M.; Sathishkumar, G.; Houshyar, S.; Senthilkumar, R.; Quigley, A.F.; Shanthakumari, S.; Padhye, R.; Bhattacharyya, A. Nanocomposite coated silk based artificial conduits: The influence of structures on regeneration of peripheral nerve. Acs Appl. Bio. Mater. 2020, 3, 4454–4464. [Google Scholar] [CrossRef]
- Nune, M.; Manchineella, S.; Govindaraju, T.; Narayan, K.S. Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering. Mater. Sci Eng. C Mater. Biol. Appl. 2019, 94, 17–25. [Google Scholar] [CrossRef]
- White, J.D.; Wang, S.; Weiss, A.S.; Kaplan, D.L. Silk–tropoelastin protein films for nerve guidance. Acta Biomater. 2015, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.J.; Yao, M.; Wang, Y.S.; Zhou, C.W.; Wan, D.Y.; Lei, P.Z.; Wen, J.; Lei, H.W.; Dong, D.M. Promotion of peripheral nerve regeneration of a peptide compound hydrogel scaffold. Int. J. Nanomed. 2013, 8, 3217–3225. [Google Scholar] [CrossRef] [Green Version]
- Dinis, T.M.; Elia, R.; Vidal, G.; Auffret, A.; Kaplan, D.L.; Egles, C. Method to form a fiber/growth factor dual-gradient along electrospun silk for nerve regeneration. Acs Appl. Mater. Interfaces 2014, 6, 16817–16826. [Google Scholar] [CrossRef] [PubMed]
- Schwarzmaier, S.M.; Kim, S.-W.; Trabold, R.; Plesnila, N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J. Neurotrauma 2010, 27, 121–130. [Google Scholar] [CrossRef]
- González-Nieto, D.; Fernández-Serra, R.; Pérez-Rigueiro, J.; Panetsos, F.; Martinez-Murillo, R.; Guinea, G.V. Biomaterials to Neuroprotect the Stroke Brain: A Large Opportunity for Narrow Time Windows. Cells 2020, 9, 1074. [Google Scholar] [CrossRef]
- Pham, D.T.; Tiyaboonchai, W. Fibroin nanoparticles: A promising drug delivery system. Drug Deliv. 2020, 27, 431–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalbán, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Karahaliloğlu, Z. Curcumin-loaded silk fibroin e-gel scaffolds for wound healing applications. Mater. Technol. 2018, 33, 276–287. [Google Scholar] [CrossRef]
- Boison, D.; Scheurer, L.; Tseng, J.L.; Aebischer, P.; Mohler, H. Seizure suppression in kindled rats by intraventricular grafting of an adenosine releasing synthetic polymer. Exp. Neurol. 1999, 160, 164–174. [Google Scholar] [CrossRef]
- Li, T.; Ren, G.; Lusardi, T.; Wilz, A.; Lan, J.Q.; Iwasato, T.; Itohara, S.; Simon, R.P.; Boison, D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J. Clin. Investig. 2008, 118, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Noebels, J.; Avoli, M.; Rogawski, M.; Olsen, R.; Delgado-Escueta, A. Jasper’s Basic Mechanisms of the Epilepsies; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Wilz, A.; Pritchard, E.M.; Li, T.; Lan, J.-Q.; Kaplan, D.L.; Boison, D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials 2008, 29, 3609–3616. [Google Scholar] [CrossRef] [Green Version]
- Szybala, C.; Pritchard, E.M.; Lusardi, T.A.; Li, T.; Wilz, A.; Kaplan, D.L.; Boison, D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp. Neurol. 2009, 219, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Boison, D.; Kaplan, D.L. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. U.S. Patent Application No. US9040073B2, 26 May 2015. [Google Scholar]
- Han, X.; Liu, H.; Kuang, X.; Wang, Z.; Wang, X. Silk fibroin improves the release of nerve growth factor from hydroxyapatite particles maintaining its bioactivity. Curr. Drug Deliv. 2018, 15, 879–886. [Google Scholar] [CrossRef]
- Dionigi, C.; Posati, T.; Benfenati, V.; Sagnella, A.; Pistone, A.; Bonetti, S.; Ruani, G.; Dinelli, F.; Padeletti, G.; Zamboni, R. A nanostructured conductive bio-composite of silk fibroin–single walled carbon nanotubes. J. Mater. Chem. B 2014, 2, 1424–1431. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, L.; Han, Q.; Chen, S.; Liu, Y.; Mu, H.; Liu, Y.; Li, G.; Chen, X.; Yang, Y. Effect of anisotropic silk fibroin topographies on dorsal root ganglion. J. Mater. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Yan, S.; Yang, Y.; Zhao, H.; Li, M.; Lu, S.; Kaplan, D.L. Preparation of uniaxial multichannel silk fibroin scaffolds for guiding primary neurons. Acta Biomater. 2012, 8, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Konwarh, R.; Gupta, P.; Mandal, B.B. Silk-microfluidics for advanced biotechnological applications: A progressive review. Biotechnol. Adv. 2016, 34, 845–858. [Google Scholar] [CrossRef]
- Kang, C.E.; Gemeinhart, E.J.; Gemeinhart, R.A. Cellular alignment by grafted adhesion peptide surface density gradients. J. Biomed. Mater. Res. Part. A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 71, 403–411. [Google Scholar] [CrossRef]
- Chelli, B.; Barbalinardo, M.; Valle, F.; Greco, P.; Bystrenova, E.; Bianchi, M.; Biscarini, F. Neural cell alignment by patterning gradients of the extracellular matrix protein laminin. Interface Focus 2014, 4, 20130041. [Google Scholar] [CrossRef]
- Moore, K.; Macsween, M.; Shoichet, M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006, 12, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodla, M.C.; Bellamkonda, R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 2008, 29, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Magaz, A.; Ashton, M.D.; Hathout, R.M.; Li, X.; Hardy, J.G.; Blaker, J.J. Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery. Pharmaceutics 2020, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chang, J.-J.; Yung, M.-C.; Huang, W.-C.; Chen, S.-Y. Spontaneously Micropatterned Silk/Gelatin Scaffolds with Topographical, Biological, and Electrical Stimuli for Neuronal Regulation. Acs Biomater. Sci. Eng. 2020, 6, 1144–1153. [Google Scholar] [CrossRef]
- Chighizola, M.; Dini, T.; Lenardi, C.; Milani, P.; Podestà, A.; Schulte, C. Mechanotransduction in neuronal cell development and functioning. Biophys. Rev. 2019, 11, 1–20. [Google Scholar] [CrossRef]
- Urbach, J. Guiding Neuronal Growth in Tissues with Light; Georgetown University: Washington, DC, USA, 2010. [Google Scholar]
- Amsden, J.J.; Kaplan, D.L.; Omenetto, F. Nanoimprinting of silk fibroin structures for biomedical and biophotonic applications. U.S. Patent Application No. US9603810B2, 28 March 2017. [Google Scholar]
- Rossitch, E., Jr.; Bullard, D.E.; Oakes, W.J. Delayed foreign-body reaction to silk sutures in pediatric neurosurgical patients. Child’s Nerv. Syst. Chns Off. J. Int. Soc. Pediatric Neurosurg. 1987, 3, 375–378. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Verma, K.; Riech, S.; Mortazavi, M.; Oakes, W.J.; Cohen-Gadol, A.A. Reaction to silk suture in children undergoing neurosurgery: Case reports and review of the literature. Child’s Nerv. Syst. 2011, 27, 497–499. [Google Scholar] [CrossRef]
- Megyesi, J.F.; Ranger, A.; MacDonald, W.; Del Maestro, R.F. Suturing Technique and the Integrity of Dural Closures: An in Vitro Study. Neurosurgery 2004, 55, 950–955. [Google Scholar] [CrossRef]
- DiMeco, F.; Li, K.W.; Mendola, C.; Cantu’, G.; Solero, C.L. Craniotomies without burr holes using an oscillating saw. Acta Neurochir. 2004, 146, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Zeplin, P.H.; Berninger, A.K.; Maksimovikj, N.C.; van Gelder, P.; Scheibel, T.; Walles, H. Improving the biocompatibility of silicone implants using spider silk coatings: Immunohistochemical analysis of capsule formation. Handchir. Mikrochir. Plast. Chir. Organ. Dtsch. Arb. Handchir. Organ. Dtsch. Arb. Mikrochir. Peripher. Nerven Gefasse 2014, 46, 336–341. [Google Scholar] [CrossRef]
- Gu, X. Progress and perspectives of neural tissue engineering. Front. Med. 2015, 9, 401–411. [Google Scholar] [CrossRef]
- Fregnan, F.; Muratori, L.; Bassani, G.A.; Crosio, A.; Biagiotti, M.; Vincoli, V.; Carta, G.; Pierimarchi, P.; Geuna, S.; Alessandrino, A. Preclinical Validation of SilkBridgeTM for Peripheral Nerve Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 835. [Google Scholar] [CrossRef] [PubMed]
- Silk Biomaterials SRL. A Pilot Study to Evaluate the Reconstruction of Digital Nerve Defects in Humans Using an Implanted Silk Nerve Guide; US National Library of Medicine: Bethesda, MD, USA, 2020.
- Johns, M. Safety and Efficacy of a Silk Protein Microparticle-based Filler for Injection Augmentation in Treating Unilateral Vocal Fold. Paralysis; US National Library of Medicine: Bethesda, MD, USA, 2021.
- Sofregen Medical Inc. A Histological Study Evaluating Silk Voice and Crosslinked Hyaluronic Acid; US National Library of Medicine: Bethesda, MD, USA, 2021.






| Biomaterial | Fabrication Method | Main Applications | Advantages | Limitations |
|---|---|---|---|---|
| Fibers | Straining Flow Spinning [52,53] | Conduits for nerves | Easy instrumental method, easy bulk and surface property modification, high-performance individual fibers | Thick diameter of fibers |
| Mats, meshes, bundles | Electrospinning [54,55,56] | Conduits for PNS damages, spinal cord injury | Fabrication of ultra-fine nanometric fibers, control of fiber properties (orientation, diameter, and composition) | Usage of organic solvents, complexity in the control of various parameters and variability |
| Custom Shapes | 3D printing [57,58] | Conduits for PNS damage, spinal cord injury | Highly reproducible, fabrication of complex 3D structures, integration with various polymers and/or cells | The nozzle and cartridges can affect cell viability, costly |
| Sponges | Solvent Casting/Porogen leaching [59,60] | Modeling polarized neural tissue Bioengineering of cortical brain tissue | Uncomplicated and easy-to-use instrumentation, coating of different biomolecules, low cost | Lack of ability to control pore communication and interpore channels |
| Lyophilization [61] | Traumatic neural tissue damage | Control the mechanical and degradation properties by initial concentration, pH, and freezing rate | Extra treatments for β-sheet-enriched conformation | |
| Hydrogels | Self-assembly [62,63] | Intracerebral applications, drug and cell delivery | No need for organic solvents or further treatment steps for induction of beta-sheet, production of ultra-fine microfibers | Complicated to define the self-assembly process, requirement of physical-chemical agents (e.g., sonication, cross-linkers) to induce gelation |
| Films | Dry Casting [35,64,65] | Drug delivery, axonal growth and guidance, neural electrodes covering | Relatively simple and low cost method, low invasiveness | Formation of films in silk I stage, necessity for further steps of treatment for induction of beta-sheet content |
| Silk Fibroin Formulated with Synthetic Materials | |||
|---|---|---|---|
| Target Cell/Tissue, In Vivo Model | Formulation | Main Results | References |
| PC12 (neural cell line) | SF/Polylactic Acid (PLA) | Elongated neurites (~95 μm), support cell attachment and differentiation | [168] |
| MSC | SF/Carbon nanotubes | Trans-differentiation towards neural cells | [169] |
| Schwann cells | SF/Gold nanofibers | Cell adhesion without toxic or immunogenic response | [170] |
| Schwann cells | SF/Graphene | Cell growth in an electroconductive and biocompatible surface | [171] |
| Neuronal progenitor cells/rat sciatic nerve model | SF/Carbon nanofibers (CNFs)/Poly-ε-caprolactone (PCL) | Cell-to-cell communication, regeneration of sciatic nerve model (~2 cm) | [172] |
| Schwann cells | SF/Polypyrrole | Arrangement of cells without toxicity | [58] |
| Silk Fibroin Formulated with Natural Materials or ECM-Derived Peptides | |||
| Target Cell, Tissue, In Vivo Model | Formulation | Main Results | References |
| Sciatic nerve injury model | SF/Chitosan | 10 mm nerve gap model bridging | [152] |
| Neuroblastoma cell line (SH-SY5Y) | SF/Melanin | Significant antioxidant potential, cell differentiation | [173] |
| NSC/rat spinal cord injury | SF/Collagen | Increasing nerve regeneration | [57] |
| Schwann cells | Silk/Tropoelastin | Cell arrangement and neurite guidance | [174] |
| MSC | SF/YIGSR and GYIGSR Integrin-binding laminin peptide motifs | Enhanced cell proliferation and differentiation | [93] |
| NSC | SF/IKVAV Integrin-binding laminin peptide motif | Improvement of cellular differentiation and viability | [90] |
| PC12/rat sciatic nerve model | SF/SF16 peptides | Enhanced cell viability and axonal growth | [175] |
| Hippocampal neurons | Silk/Laminin | Stimulation of cell growth, differentiation, and neurite extension | [162] |
| Silk Fibroin Formulated with Growth Factors | |||
| Target Cell, Tissue, In Vivo Model | Formulation | Main Results | References |
| PC12 | SF/PLA/NGF | Sustained release of NGF, increased neurite outgrowth (~95 μm) | [168] |
| Rat dorsal root ganglion neurons (DRG) | SF/NGF (gradient distribution) | Cell growth and orientation (NGF gradient) | [176] |
| DRG | SF/NGF/CNTF | Enhancement of neurite outgrowth | [106] |
| Schwann Cells | SF/BDNF/VEGF | Improvement of cell growth and vascularization | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonesi, M.; Garcia-Nieto, M.; Guinea, G.V.; Panetsos, F.; Pérez-Rigueiro, J.; González-Nieto, D. Silk Fibroin: An Ancient Material for Repairing the Injured Nervous System. Pharmaceutics 2021, 13, 429. https://doi.org/10.3390/pharmaceutics13030429
Yonesi M, Garcia-Nieto M, Guinea GV, Panetsos F, Pérez-Rigueiro J, González-Nieto D. Silk Fibroin: An Ancient Material for Repairing the Injured Nervous System. Pharmaceutics. 2021; 13(3):429. https://doi.org/10.3390/pharmaceutics13030429
Chicago/Turabian StyleYonesi, Mahdi, Mario Garcia-Nieto, Gustavo V. Guinea, Fivos Panetsos, José Pérez-Rigueiro, and Daniel González-Nieto. 2021. "Silk Fibroin: An Ancient Material for Repairing the Injured Nervous System" Pharmaceutics 13, no. 3: 429. https://doi.org/10.3390/pharmaceutics13030429