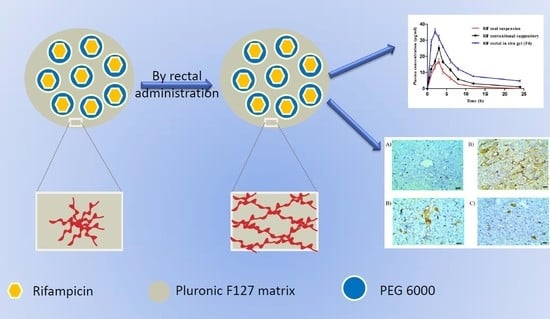
Mucoadhesive In Situ Rectal Gel Loaded with Rifampicin: Strategy to Improve Bioavailability and Alleviate Liver Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of RF Co-Precipitate
2.3. Determination of RF Co-Precipitate Aqueous Solubility
2.4. RF/PEG 6000 Interaction
2.4.1. Thermal Analysis Using Differential Scanning Calorimeter (DSC)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Preparation of in Situ Gels
2.6. Gelation Temperature
2.7. Gel Strength Determination
2.8. Animals
2.9. In Vivo Rectal Retention
2.10. Investigation of Rectal Irritation
2.11. Pharmacokinetics Study
2.12. Chromatographic Conditions
2.13. Sample Preparation
2.14. Toxicity Studies
2.15. Statistical Analysis
3. Results and Discussion
3.1. RF Co-Precipitate Aqueous Solubility
3.2. Thermal Analysis
3.3. FTIR Analysis
3.4. Gelation Temperature
3.5. Gel Strength Determination
3.6. Rectal Retention
3.7. Rectal Irritation
3.8. Pharmacokinetic Studies
3.9. Toxicity
3.9.1. Biochemical Changes
3.9.2. Histopathological Examination
3.9.3. Immunohistochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moretton, M.A.; Glisoni, R.J.; Chiappetta, D.A.; Sosnik, A. Molecular implications in the nanoencapsulation of the anti-tuberculosis drug rifampicin within flower-like polymeric micelles. Colloids Surf. B Biointerfaces 2010, 79, 467–479. [Google Scholar] [CrossRef]
- Annabel, B.; Anna, D.; Hannah, M. Global Tuberculosis Report Geneva World Heal Organ; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Du Toit, L.C.; Pillay, V.; Danckwerts, M.P. Tuberculosis chemotherapy: Current drug delivery approaches. Respir. Res. 2006, 7, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, N.; Barau, C.; Amin, A.; Baudin, E.; Meggi, B.; Silva, C.; Furlan, V.; Grinsztejn, B.; Barrailtran, A.; Bonnet, M.; et al. Pharmacokinetics of Rifampin and Isoniazid in Tuberculosis-HIV-Coinfected Patients Receiving Nevirapine- or Efavirenz-Based Antiretroviral Treatment. Antimicrob. Agents Chemother. 2014, 58, 3182–3190. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.; Bhatt, T.D.; Gill, M.S.; Suresh, S. Novel rifampicin–phospholipid complex for tubercular therapy: Synthesis, physico-chemical characterization and in-vivo evaluation. Int. J. Pharm. 2014, 460, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, S.S.; Dighe, V.D.; Kotak, D.; Devarajan, P.V. Rifampicin Lipid-Polymer hybrid nanoparticles (LIPOMER) for enhanced Peyer’s patch uptake. Int. J. Pharm. 2017, 532, 612–622. [Google Scholar] [CrossRef]
- Singh, C.; Koduri, L.S.K.; Dhawale, V.; Bhatt, T.D.; Kumar, R.; Grover, V.; Tikoo, K.; Suresh, S. Potential of aerosolized rifampicin lipospheres for modulation of pulmonary pharmacokinetics and bio-distribution. Int. J. Pharm. 2015, 495, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Koduri, L.S.K.; Kumar, U.A.; Bhatt, T.D.; Kumar, V.; Sobhia, M.E.; Suresh, S. Attenuation potential of rifampicin–phospholipid complex in murine hepatotoxicity model. J. Drug Deliv. Sci. Technol. 2015, 30, 225–231. [Google Scholar] [CrossRef]
- Xuan, J.J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Yong, C.S.; Kim, H.M.; Rhee, J.D.; Oh, Y.K.; Kim, C.K.; Choi, H.G. Effect of sodium chloride on the release, absorption and safety of diclofenac sodium delivered by poloxamer gel. Int. J. Pharm. 2003, 263, 105–111. [Google Scholar] [CrossRef]
- Kim, C.K.; Lee, S.W.; Choi, H.G.; Lee, M.K.; Gao, Z.G.; Kim, I.S.; Park, K.M. Trials of in situ-gelling and mucoadhesive acetaminophen liquid suppository in human subjects. Int. J. Pharm. 1998, 174, 201–207. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological charac-teristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Liu, Y.; Di, X. Thermosensitive in situ gel based on solid dispersion for rectal delivery of ibuprofen. AAPS Pharm. Sci. Tech. 2018, 19, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.P.; Guo, Y.S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef]
- Din, F.U.; Mustapha, O.; Kim, D.W.; Rashid, R.; Park, J.H.; Choi, J.Y.; Ku, S.K.; Yong, C.S.; Kim, J.O.; Choi, H.G. Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. Eur. J. Pharm. Biopharm. 2015, 94, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Kim, C.K. Design and evaluation of ondansetron liquid suppository for the treatment of emesis. Arch. Pharmacal. Res. 2013, 36, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Cafaggi, S.; Russo, E.; Caviglioli, G.; Parodi, B.; Stefani, R.; Sillo, G.; Leardi, R.; Bignardi, G. Poloxamer 407 as a solubilising agent for tolfenamic acid and as a base for a gel formulation. Eur. J. Pharm. Sci. 2008, 35, 19–29. [Google Scholar] [CrossRef]
- Yun, M.; Choi, H.; Jung, J.; Kim, C. Development of a thermo-reversible insulin liquid suppository with bioavailability enhancement. Int. J. Pharm. 1999, 189, 137–145. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Salama, A.; Soliman, G.M.; Alruwaili, N.K.; Mostafa, E.M.; Mohammed, E.F.; Moustafa, A.E.G.A.; Zafar, A. Soy isoflavone-loaded alginate micro-spheres in thermosensitive gel base: Attempts to improve wound-healing efficacy. J. Pharm. Pharmacol. 2019, 71, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.S. In Vitro and In Vivo Characteristics of a Thermogelling Rectal Delivery System of Etodolac. AAPS PharmSciTech 2009, 10, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.G.; Oh, Y.K.; Kim, C.K. In situ gelling and mucoadhesive liquid suppository containing acetaminophen: Enhanced bio-availability. Int. J. Pharm. 1998, 165, 23–32. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Badran, M.M.; Ali, H.M.; Bakky, A.M.S.; Ibrahim, H.M. Multifunctional carbamazepine loaded nanostructured lipid carrier (NLC) formulation. Int. J. Pharm. 2018, 550, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Li, J.J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Ashokraj, Y.; Bharatam, P.V.; Pillai, O.; Panchagnula, R. Solid-state characterization of rifampicin samples and its biopharmaceutic relevance. Eur. J. Pharm. Sci. 2004, 22, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.; Patel, S.B. Phase behavior, intermolecular interaction, and solid state characterization of amorphous solid dispersion of Febuxostat. Pharm. Dev. Technol. 2016, 22, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.A.; Vahur, S.; Leito, I. ATR-FTIR spectroscopy and quantitative multivariate analysis of paints and coating materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Soon, Y.C.; Kwon, Y.C.; Yong, I.I.K.; Joo, B.P.; Zhe, Q.Q.; Dai, J.R.; Kook, K.C.; Gon, C.H. Physicochemical characterization andin vivo evaluation of thermosensitive diclofenac liquid suppository. Arch. Pharm. Res. 2003, 26, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Carlfors, J.; Petersson, R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; El Hady, S.S.A. Enhanced bioavailability of metoclopramide HCl by intranasal administra-tion of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Biondi, M.; Quaglia, F.; Fusco, S.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. Injectable Thermally Responsive Mucoadhesive Gel for Sustained Protein Delivery. Biomacromolecules 2011, 12, 28–33. [Google Scholar] [CrossRef]
- Yong, C.S.; Choi, J.S.; Quan, Q.Z.; Rhee, J.D.; Kim, C.K.; Lim, S.J.; Kim, K.M.; Oh, P.S.; Choi, H.G. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2001, 226, 195–205. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Ryu, J.M.; Chung, S.J.; Lee, M.H.; Kim, C.K.; Shim, C.K. Increased bioavailability of propranolol in rats by retaining thermally gelling liquid suppositories in the rectum. J. Control. Release 1999, 59, 163–172. [Google Scholar] [CrossRef]
- Sankar, R.; Sharda, N.; Singh, S. Behavior of Decomposition of Rifampicin in the Presence of Isoniazid in the pH Range 1. Drug Dev. Ind. Pharm. 2003, 29, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Le Ray, A.M.; Iooss, P.; Gouyette, A.; Vonarx, V.; Patrice, T.; Merle, C. Development of a “continuous-flow adhesion cell” for the assessment of hydrogel adhesion. Drug Dev. Ind. Pharm. 1999, 25, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, L.E. Interactions among proteins and hydrophobically modified polyelectrolytes. J. Pharm. Pharmacol. 2001, 53, 541–547. [Google Scholar] [CrossRef]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Ola, R.P.; Singh, K. Hepatoprotection by carotenoids in isoniazid–rifampicin induced hepatic injury in rats. Biochem. Cell Biol. 2010, 88, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Bigoniya, P.; Singh, C.S.; Shukla, A. A comprehensive review of different liver toxicants used in experimental pharmacology. Int. J. Pharm. Sci. Drug Res. 2009, 1, 124–135. [Google Scholar]
- Santhosh, S.; Sini, T.K.; Anandan, R.; Mathew, P.T. Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur. J. Pharmacol. 2007, 572, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Gopi, M.; Seshadri, M.S. Biphasic Effect of Rifampicin on Bilirubin- A Case Report. J. Clin. Diagn. Res. 2016, 10, 7614. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K.; Vanithakumari, G.; Indra, N. Effect of rifampicin on certain biochemical parameter in the liver of albino rats. Internet J. Toxicol. 2009. [Google Scholar] [CrossRef]
- Rafiq, S.; Iqbal, T.; Jamil, A.; Khan, F.H. Pharmacokinetic studies of rifampicin in healthy volunteers and tuberculosis patients. Int. J. Agric. Biol. 2010, 12, 391–395. [Google Scholar]
- Chen, X.; Zhang, C.; Wang, H.; Xu, J.; Duan, Z.H.; Zhang, Y.; Yu, T.; Wei, W.; Xu, D.X.; Xu, J.M. Altered integrity and decreased expression of hepatocyte tight junctions in rifampicin-induced cholestasis in mice. Toxicol. Appl. Pharmacol. 2009, 240, 26–36. [Google Scholar] [CrossRef]
- Tostmann, A.; Boeree, M.J.; E Aarnoutse, R.; De Lange, W.C.M.; Ven, A.J.A.M.V.D.; Dekhuijzen, R. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroenterol. Hepatol. 2008, 23, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Zhang, C.; Zhang, D.G.; Li, L.; Chen, X.; Xu, D.X. Rifampicin-Induced Hepatic Lipid Accumulation: Association with Up-Regulation of Peroxisome Proliferator-Activated Receptor γ in Mouse Liver. PLoS ONE 2016, 11, 5787. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Sawairi, M.; Nakagawa, M.; Itoh, N.; Wada, K.; Tamaya, T. Peritoneal Fluid lnterleukin-1β and Tumor Ne-crosis Factor in Patients with Benign Gynecologic Disease. Am. J. Reprod. Immunol. 1991, 26, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, N.F.; Junior, R.V.; Santos, A.A., Jr.; Leite, C.E.; Dias, A.C.O.; Batista, E.L., Jr.; Basso, L.A.; Campos, M.M.; Santos, D.S.; Souto, A.A. Protective effects of resveratrol on hepatotoxicity induced by isoniazid and rifampicin via SIRT1 modulation. J. Nat. Prod. 2014, 77, 2190–2195. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Yoon, H.J.; Shin, D.H.; Park, S.S.; Kim, Y.; Park, J.S.; Jee, Y.K. TNF-α genetic polymorphism− 308G/A and antituberculosis drug-induced hepatitis. Liver Int. 2012, 32, 809–814. [Google Scholar] [CrossRef]









| Code | Base | Co-Precipitate % | Sodium Alginate % | HPMC % | Chitosan % | |
|---|---|---|---|---|---|---|
| Pluronic F127% | Pluronic F68% | |||||
| F1 | 15 | 10 | 0.1 | --- | --- | --- |
| F2 | 15 | 10 | 0.1 | 0.4 | --- | --- |
| F3 | 15 | 10 | 0.1 | 0.8 | --- | --- |
| F4 | 15 | 10 | 0.1 | 1.2 | --- | --- |
| F5 | 15 | 10 | 0.1 | --- | 0.5 | --- |
| F6 | 15 | 10 | 0.1 | --- | 1 | --- |
| F7 | 15 | 10 | 0.1 | --- | 1.5 | --- |
| F8 | 15 | 10 | 0.1 | --- | --- | 0.5 |
| Code | Tsol-gel (°C) ± SD | Gel Strength (s) ± SD | |
|---|---|---|---|
| Base | 36.1 ± 1.5 | 21.5 ± 1.6 | |
| F1 | 43.8 ± 3.8 | 22.4 ± 3.5 | |
| F2 | 40.5 ± 1.9 | 27.6 ± 0.7 | |
| F3 | 38.5 ± 2.1 | 34 ± 1.8 | |
| F4 | 34.2 ± 2.7 | 41.5 ± 2.8 | |
| F5 | 41.3 ± 1.5 | 29.5 ± 0.9 | |
| F6 | 37.8 ± 1.6 | 35.9 ± 1.7 | |
| F7 | Gel formed at room temperature | 47.8 ± 3.8 | |
| F8 | ppt. formed | - |
| Parameter | Oral RF Suspension | Rectal RF Solid Suppository | Rectal RF in Situ Gel (F4) |
|---|---|---|---|
| Tmax (h) | 3 | 3 | 2 |
| Cmax (µg/mL) | 16.9 ± 2.9 | 25.1 ± 2.7 | 35.5 ± 3.1 |
| T1/2ab (h) | 1.07 ± 0.93 | 0.15 ± 0.07 | 4.95 ± 0.35 |
| T1/2el (h) | 6.35 ± 2.13 | 5.20 ± 1.0 | 7.96 ± 0.8 |
| AUC0–24 (µg·h/mL) | 86.76 ± 6.8 | 151.70 ± 12.4 | 293.76 ± 21.7 |
| MRT (h) | 5.38 ± 0.84 | 6.54 ± 0.27 | 7.86 ± 1.1 |
| RB | -------- | 1.74 | 3.38 |
| Biochemical Parameter | Control | Oral RF Suspension | Rectal RF Conventional Suppository | Rectal RF in Situ Gel (F4) |
|---|---|---|---|---|
| ALT (U/L) | 48.04 ± 3.8 | 80.30 ± 0.16 | 57.30 ± 4.3 | 50.0 ± 3.9 |
| AST (U/L) | 12.71 ± 1.3 | 37.97 ± 5.9 | 32.71 ± 4.4 | 22.26 ± 1.7 |
| Albumin (mg/dL) | 4.26 ± 0.35 | 3.41 ± 0.18 | 3.80 ± 0.14 | 3.88 ± 0.16 |
| Total bilirubin (mg/dL) | 0.22 ± 0.02 | 0.61 ± 0.06 | 0.35 ± 0.05 | 0.26 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Joufi, F.; Elmowafy, M.; Alruwaili, N.K.; Alharbi, K.S.; Shalaby, K.; Alsharari, S.D.; Ali, H.M. Mucoadhesive In Situ Rectal Gel Loaded with Rifampicin: Strategy to Improve Bioavailability and Alleviate Liver Toxicity. Pharmaceutics 2021, 13, 336. https://doi.org/10.3390/pharmaceutics13030336
Al-Joufi F, Elmowafy M, Alruwaili NK, Alharbi KS, Shalaby K, Alsharari SD, Ali HM. Mucoadhesive In Situ Rectal Gel Loaded with Rifampicin: Strategy to Improve Bioavailability and Alleviate Liver Toxicity. Pharmaceutics. 2021; 13(3):336. https://doi.org/10.3390/pharmaceutics13030336
Chicago/Turabian StyleAl-Joufi, Fakhria, Mohammed Elmowafy, Nabil K. Alruwaili, Khalid S. Alharbi, Khaled Shalaby, Shakir D. Alsharari, and Hazim M. Ali. 2021. "Mucoadhesive In Situ Rectal Gel Loaded with Rifampicin: Strategy to Improve Bioavailability and Alleviate Liver Toxicity" Pharmaceutics 13, no. 3: 336. https://doi.org/10.3390/pharmaceutics13030336