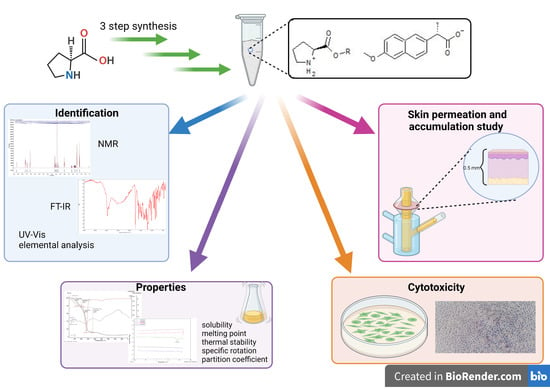
Novel Naproxen Salts with Increased Skin Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Skin
2.3. Synthesis of the L-Proline Alkyl Ester Naproxenate [ProOR][NAP]
2.4. General Analytical Methods
2.4.1. Identification and Properties of [ProOR][NAP]
2.4.2. Methodology of Skin Permeation and Accumulation
2.4.3. HPLC
2.4.4. Cytotoxicity Experiments
2.4.5. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Identification of [ProOR][NAP]




3.1.1. Comparison of NMR Spectra for [ProOR][NAP]
3.1.2. Comparison of FTIR Spectra for [ProOR][NAP]
3.2. X-ray Powder Diffraction
3.3. Physicochemical Properties of [ProOR][NAP]
3.4. Skin Permeation and Skin Accumulation of Naproxen and Its Salts with L-Proline Esters
3.5. Cytotoxicity of Naproxen and Its Salts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haznar-Garbacz, D.; Polak, S. Physiological factors affecting the bioavailability of the drugs. Farm. Pol. 2011, 67, 459–470. [Google Scholar]
- Currie, G.M. Pharmacology, Part 2: Introduction to Pharmacokinetics. J. Nucl. Med. Technol. 2018, 46, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, M. Factors Affecting the Bioavailability of Chemicals. In An Introduction to Aquatic Toxicology; Nikinmaa, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 65–72. [Google Scholar]
- Atkinson, A.J. Drug Absorption and Bioavailability. In Principles of Clinical Pharmacology, 3rd ed.; Huang, S.-M., Lertora, J., Atkinson, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 37–49. [Google Scholar]
- Rannou, F.; Pelletier, J.-P.; Martel-Pelletier, J. Efficacy and Safety of Topical NSAIDs in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Semin. Arthritis Rheum. 2016, 45, S18–S21. [Google Scholar] [CrossRef] [Green Version]
- Newa, M.; Bhandari, K.H.; Kim, J.O.; Im, J.S.; Kim, J.A.; Yoo, B.K.; Woo, J.S.; Choi, H.G.; Yong, C.S. Enhancement of Solubility, Dissolution and Bioavailability of Ibuprofen in Solid Dispersion Systems. Chem. Pharm. Bull. 2008, 56, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartlieb, K.J.; Ferris, D.P.; Holcroft, J.M.; Kandela, I.; Stern, C.L.; Nassar, M.S.; Botros, Y.Y.; Stoddart, J.F. Encapsulation of Ibuprofen in CD-MOF and Related Bioavailability Studies. Mol. Pharm. 2017, 14, 1831–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, D.D.; Karara, A.H. Characterization, Dissolution and Bioavailability in Rats of Ibuprofen-β-Cyclodextrin Complex System. Int. J. Pharm. 1986, 28, 95–101. [Google Scholar] [CrossRef]
- Li, D.X.; Oh, Y.-K.; Lim, S.-J.; Kim, J.O.; Yang, H.J.; Sung, J.H.; Yong, C.S.; Choi, H.-G. Novel Gelatin Microcapsule with Bioavailability Enhancement of Ibuprofen Using Spray-Drying Technique. Int. J. Pharm. 2008, 355, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.S.; Yang, C.H.; Rhee, J.-D.; Lee, B.-J.; Kim, D.-C.; Kim, D.-D.; Kim, C.-K.; Choi, J.-S.; Choi, H.-G. Enhanced Rectal Bioavailability of Ibuprofen in Rats by Poloxamer 188 and Menthol. Int. J. Pharm. 2004, 269, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, L.K.; Ghosh, N.C.; Chatterjee, M.; Gupta, B.K. Product Development Studies on the Tablet Formulation of Ibuprofen to Improve Bioavailability. Drug Dev. Ind. Pharm. 1998, 24, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.; Afrose, A.; Islam, N. Formulation and Delivery Strategies of Ibuprofen: Challenges and Opportunities. Drug Dev. Ind. Pharm. 2018, 44, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Forsgren, J.; Strømme, M. Stabilisation of Amorphous Ibuprofen in Upsalite, a Mesoporous Magnesium Carbonate, as an Approach to Increasing the Aqueous Solubility of Poorly Soluble Drugs. Int. J. Pharm. 2014, 472, 185–191. [Google Scholar] [CrossRef]
- Kumar, L.; Suhas, B.; Pai, G.K.; Verma, R. Determination of Saturated Solubility of Naproxen Using UV Visible Spectrophotometer. Res. J. Pharm. Technol. 2015, 8, 825. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Al-Hamad, S.S.; Lee, K.; Li, N.; Gary, B.D.; Keeton, A.B.; Piazza, G.A.; Abdel-Hamid, M.K. Novel Non-Cyclooxygenase Inhibitory Derivatives of Naproxen for Colorectal Cancer Chemoprevention. Med. Chem. Res. 2014, 23, 4177–4188. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Löbmann, K.; Rades, T.; Grohganz, H. Improving Co-Amorphous Drug Formulations by the Addition of the Highly Water Soluble Amino Acid, Proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Lobo, L.; Dengale, S.; Grohganz, H.; Rades, T.; Löbmann, K. In Vitro and in Vivo Comparison between Crystalline and Co-Amorphous Salts of Naproxen-Arginine. Eur. J. Pharm. Biopharm. 2018, 132, 192–199. [Google Scholar] [CrossRef]
- Mura, P. Ternary Systems of Naproxen with Hydroxypropyl-β-Cyclodextrin and Aminoacids. Int. J. Pharm. 2003, 260, 293–302. [Google Scholar] [CrossRef]
- Mura, P.; Bettinetti, G.P.; Cirri, M.; Maestrelli, F.; Sorrenti, M.; Catenacci, L. Solid-State Characterization and Dissolution Properties of Naproxen–Arginine–Hydroxypropyl-β-Cyclodextrin Ternary System. Eur. J. Pharm. Biopharm. 2005, 59, 99–106. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic Liquids for Clean Technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, J.A.P. Group Contribution Methods for the Prediction of Thermophysical and Transport Properties of Ionic Liquids. AIChE J. 2009, 55, 1274–1290. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, A.M.O.; Costa, S.P.F.; Dias, A.F.V.; Marques, A.H.O.; Pinto, P.C.A.G.; Bica, K.; Ressmann, A.K.; Passos, M.L.C.; Araújo, A.R.T.S.; Reis, S.; et al. Anti-Inflammatory Choline Based Ionic Liquids: Insights into Their Lipophilicity, Solubility and Toxicity Parameters. J. Mol. Liq. 2017, 232, 20–26. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Woo Kim, S.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and Hydroxyproline Metabolism: Implications for Animal and Human Nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and Functional Comparisons of Four Anatomical Regions of Porcine Skin with Human Abdominal Skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine Ear Skin: An in Vitro Model for Human Skin. Ski. Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Badran, M.M.; Kuntsche, J.; Fahr, A. Skin Penetration Enhancement by a Microneedle Device (Dermaroller®) in Vitro: Dependency on Needle Size and Applied Formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® Synthetic Membrane: Permeability Comparison to Human Cadaver Skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell Using Synthetic Membranes and Pig Ear Skin with in Vivo-in Vitro Correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Ossowicz, P.; Klebeko, J.; Janus, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. The Effect of Alcohols as Vehicles on the Percutaneous Absorption and Skin Retention of Ibuprofen Modified with l -Valine Alkyl Esters. RSC Adv. 2020, 10, 41727–41740. [Google Scholar] [CrossRef]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-Species Assessment of Electrical Resistance as a Skin Integrity Marker for in Vitro Percutaneous Absorption Studies. Toxicol. Vitr. 2004, 18, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ossowicz, P.; Kardaleva, P.; Guncheva, M.; Klebeko, J.; Świątek, E.; Janus, E.; Yancheva, D.; Angelov, I. Ketoprofen-Based Ionic Liquids: Synthesis and Interactions with Bovine Serum Albumin. Molecules 2020, 25, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossowicz, P.; Janus, E.; Klebeko, J.; Światek, E.; Kardaleva, P.; Taneva, S.; Krachmarova, E.; Rangelov, M.; Todorova, N.; Guncheva, M. Modulation of the Binding Affinity of Naproxen to Bovine Serum Albumin by Conversion of the Drug into Amino Acid Ester Salts. J. Mol. Liq. 2020, 319, 114283. [Google Scholar] [CrossRef]
- Furniss, B.S.; Vogel, A.I. (Eds.) Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Pearson/Prentice Hall: Harlow, UK, 2009; ISBN 978-0-582-46236-6. [Google Scholar]
- International Organization for Standardization. ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Holbeck, S.L.; Collins, J.M.; Doroshow, J.H. Analysis of Food and Drug Administration–Approved Anticancer Agents in the NCI60 Panel of Human Tumor Cell Lines. Mol. Cancer Ther. 2010, 9, 1451–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004; pp. 1–25. [Google Scholar]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of Ibuprofen Solubility and Skin Permeation by Conjugation with L-Valine Alkyl Esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [Green Version]
- Ossowicz, P.; Janus, E.; Schroeder, G.; Rozwadowski, Z. Spectroscopic Studies of Amino Acid Ionic Liquid-Supported Schiff Bases. Molecules 2013, 18, 4986–5004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozwadowski, Z. Deuterium Isotope Effects on 13C Chemical Shifts of Lithium Salts of Schiff Bases Amino Acids. J. Mol. Struct. 2005, 753, 127–131. [Google Scholar] [CrossRef]
- Breitmaier, E.; Voelter, W. 13C-NMR Spectroscopy, Methods and Applications in Organic Chemistry. Monographs in Modern Chemistry, 2nd ed.; Chemie: Weinheim, Germany; New York, NY, USA, 1978; Volume 5. [Google Scholar]
- Vairam, S.; Premkumar, T.; Govindarajan, S. Trimellitate Complexes of Divalent Transition Metals with Hydrazinium Cation: Thermal and Spectroscopic Studies. J. Therm. Anal. Calorim. 2010, 100, 955–960. [Google Scholar] [CrossRef]
- Kolev, T.; Spiteller, M.; Koleva, B. Spectroscopic and Structural Elucidation of Amino Acid Derivatives and Small Peptides: Experimental and Theoretical Tools. Amino Acids 2010, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R. A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures. N.P.G. Roeges. J. Am. Chem. Soc. 1996, 118, 3543. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic Liquids. In Encyclopedia of Analytical Science. Chemistry Molecular Sciences and Engineering, 3rd ed.; Davies, P., Manisha, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 218–225. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Jedynak, K.; Szczepanik, B.; Rędzia, N.; Słomkiewicz, P.; Kolbus, A.; Rogala, P. Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions. Water 2019, 11, 1099. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Cabeza, A.J. Acid-base crystalline complexes and the pKa rule. Cryst. Eng. Comm. 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Brittain, H.G. Strategy for the Prediction and Selection of Drug Substance Salt Forms. Pharm. Technol. 2007, 31, 78–88. [Google Scholar]
- Welton, T. Ionic Liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Disasa Irge, D. Ionic Liquids: A Review on Greener Chemistry Applications, Quality Ionic Liquid Synthesis and Economical Viability in a Chemical Processes. AJPC 2016, 5, 74. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Rostamkalaei, S.S.; Asadi, M.; Asare-Addo, K.; Nokhodchi, A. The Design of Naproxen Solid Lipid Nanoparticles to Target Skin Layers. Colloids Surf. B Biointerfaces 2016, 145, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. In Vivo Skin Permeation of Sodium Naproxen Formulated in Pluronic F-127 Gels: Effect of Azone® and Transcutol®. Drug Dev. Ind. Pharm. 2005, 31, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Hasenkopf, K.; Eisner, P.; Kerscher, M. Release and in Vitro Skin Permeation of Polyphenols from Cosmetic Emulsions. Int. J. Cosmet. Sci. 2013, 35, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Atta-ur-Rahman, W.; Caldwell, G.; Iqbal Choudhary, M.; Yan, Z. (Eds.) Frontiers in Drug Design & Discovery (Volume 4); Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; ISBN 978-1-60805-202-8. [Google Scholar]
- Benson, H. Transdermal Drug Delivery: Penetration Enhancement Techniques. CDD 2005, 2, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.I.J.; McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Innovative Strategies for Enhancing Topical and Transdermal Drug Delivery. TODDJ 2007, 1, 36–59. [Google Scholar] [CrossRef]
- Sarveiya, V.; Templeton, J.F.; Benson, H.A.E. Ion-Pairs of Ibuprofen: Increased Membrane Diffusion. J. Pharm. Pharmacol. 2010, 56, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Meidan, V.M.; Horwitz, E. Methods for Quantitative Determination of Drug Localized in the Skin. J. Control. Release 1998, 56, 7–21. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Sig. Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Hughes, A.; Saunders, F.R.; Wallace, H.M. Naproxen Causes Cytotoxicity and Induces Changes in Polyamine Metabolism Independent of Cyclo-Oxygenase Expression. Toxicol. Res. 2012, 1, 108. [Google Scholar] [CrossRef]
- ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 18 November 2021).
















| Compound | Yield (%) | State |
|---|---|---|
| [ProOEt][NAP] | 98.33 ± 0.47 | white solid |
| [ProOPr][NAP] | 98.00 ± 0.82 | white solid |
| [ProOiPr][NAP] | 97.67 ± 0.94 | white solid |
| [ProOBu][NAP] | 98.67 ± 0.47 | white solid |
| Compound | Molar Mass (g mol−1) | Tm (°C) | Tonset (°C) | Tmax (°C) | Specific Rotation | Molar Specific Rotation |
|---|---|---|---|---|---|---|
| Naproxen | 230.260 | 156.37 | 249.9 | 280.7 | +50.99 | +117.41 |
| [ProOEt][NAP] | 373.443 | 91.15 | 107.3 | 273.7 | +5.660 | +21.137 |
| [ProOPr][NAP] | 387.469 | 70.05 | 108.4 | 290.1 | +4.200 | +16.274 |
| [ProOiPr][NAP] | 387.469 | 72.96 | 99.6 | 273.0 | +1.775 | +6.878 |
| [ProOBu][NAP] | 401.496 | 68.73 | 114.0 | 276.4 | +2.740 | +11.001 |
| Compound | pKa of the Acid | Log(Ks) | Percent Salt Formed |
|---|---|---|---|
| [ProOEt][NAP] | 9.63 | 5.48 | >99 |
| [ProOPr][NAP] | 9.95 | 5.80 | >99 |
| [ProOiPr][NAP] | 9.75 | 5.60 | >99 |
| [ProOBu][NAP] | 9.97 | 5.82 | >99 |
| Compound | Water (63.1) | Ethanol (51.9) | DMSO (45.1) | Chloroform (39.1) | Ethyl Acetate (38.1) | Diethyl Ether (34.5) | Toluene (33.9) | n-Hexane (31.0) |
|---|---|---|---|---|---|---|---|---|
| Naproxen | - | + | + | +/- | +/- | +/- | - | - |
| [ProOEt][NAP] | - | + | + | + | - | - | - | - |
| [ProOPr][NAP] | - | + | + | + | - | - | - | - |
| [ProOiPr][NAP] | - | + | + | + | - | - | - | - |
| [ProOBu][NAP] | - | + | + | + | - | - | - | - |
| Compound | Solubility in Water at 25 °C | Solubility in PBS (pH 7.4) at 32 °C | ||
|---|---|---|---|---|
| g dm−3 | g NAP dm−3 | g dm−3 | g NAP dm−3 | |
| Naproxen | 0.2699 ± 0.0008 | 0.2699 ± 0.0008 | 1.6936 ± 0.0036 | 1.6936 ± 0.0036 |
| [ProOEt][NAP] | 3.7924 ± 0.0129 | 2.3383 ± 0.0080 | 5.113 ± 0.0209 | 3.153 ± 0.1289 |
| [ProOPr][NAP] | 4.1099 ± 0.0103 | 2.4424 ± 0.0061 | 5.742 ± 0.0080 | 3.412 ±0.0048 |
| [ProOiPr][NAP] | 4.7646 ± 0.0050 | 2.8314 ± 0.0030 | 5.249 ± 0.0319 | 3.119 ± 0.0190 |
| [ProOBu][NAP] | 4.4698 ± 0.0050 | 2.5635 ± 0.0029 | 5.995 ± 0.0765 | 3.438 ± 0.0439 |
| Compound | logP |
|---|---|
| Naproxen | 2.117 ± 0.0097 |
| [ProOEt][NAP] | 0.898 ± 0.0148 |
| [ProOPr][NAP] | 0.987 ± 0.0189 |
| [ProOiPr][NAP] | 0.921 ± 0.0373 |
| [ProOBu][NAP] | 1.094 ± 0.0218 |
| Cumulative Permeation Mass (µg·cm−2) | [NAP] | [ProOEt][NAP] | [ProOPr][NAP] | [ProOiPr][NAP] | [ProOBu][NAP] |
| 45.55 ± 1.89 a | 277.38 ± 25.438 b | 386.02 ± 1.574 c | 389.12 ± 13.031 c | 368.70 ± 13.848 c |
| [NAP] | [ProOEt][NAP] | [ProOPr][Nap] | [ProOiPr][NAP] | [ProOBu][NAP] | |
|---|---|---|---|---|---|
| [NAP] | Z = −3.3081 p = 0.000 * | Z = −3.3081 p = 0.000 * | Z = −3.3081 p = 0.000 * | Z = −3.3081 p = 0.000 * | |
| [ProOEt][NAP] | Z = −0.4725 p = 0.6363 | Z = 1.5228 p = 0.1278 | Z = −0.4725 p = 0.6363 | ||
| [ProOPr][Nap] | Z = 0.2625 p = 0.7928 | Z = −0.0525 p = 0.9581 | |||
| [ProOiPr][NAP] | Z = 0.2625 p = 0.7928 |
| Compound | JSS, µg cm−2 h−1 | KP∙103, cm/h | LT, h | D∙104, cm2/h | Km | Q%24 h | EF |
|---|---|---|---|---|---|---|---|
| Naproxen | 7.778 | 3.111 | 0.460 | 9.066 | 0.172 | 1.822 | 1 |
| [ProOEt][NAP] | 21.854 | 8.345 | 1.316 | 3.167 | 1.318 | 6.531 | 2.682 |
| [ProOPr][NAP] | 29.158 | 12.247 | 1.431 | 2.911 | 2.104 | 9.635 | 3.936 |
| [ProOiPr][NAP] | 29.753 | 11.457 | 0.809 | 5.149 | 1.113 | 8.905 | 3.683 |
| [ProOBu][NAP] | 23.448 | 10.056 | 0.787 | 5.294 | 0.950 | 9.069 | 3.232 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świątek, E.; Ossowicz-Rupniewska, P.; Janus, E.; Nowak, A.; Sobolewski, P.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Novel Naproxen Salts with Increased Skin Permeability. Pharmaceutics 2021, 13, 2110. https://doi.org/10.3390/pharmaceutics13122110
Świątek E, Ossowicz-Rupniewska P, Janus E, Nowak A, Sobolewski P, Duchnik W, Kucharski Ł, Klimowicz A. Novel Naproxen Salts with Increased Skin Permeability. Pharmaceutics. 2021; 13(12):2110. https://doi.org/10.3390/pharmaceutics13122110
Chicago/Turabian StyleŚwiątek, Ewelina, Paula Ossowicz-Rupniewska, Ewa Janus, Anna Nowak, Peter Sobolewski, Wiktoria Duchnik, Łukasz Kucharski, and Adam Klimowicz. 2021. "Novel Naproxen Salts with Increased Skin Permeability" Pharmaceutics 13, no. 12: 2110. https://doi.org/10.3390/pharmaceutics13122110