Tablets of “Hydrochlorothiazide in Cyclodextrin in Nanoclay”: A New Nanohybrid System with Enhanced Dissolution Properties
Abstract
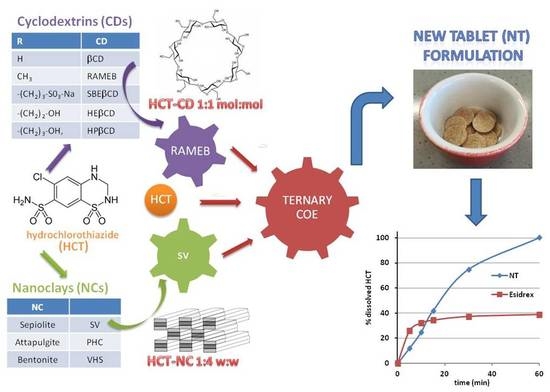
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phase Solubility Studies
2.3. Preparation of Drug–CD and Drug–NC Binary Systems
2.4. Preparation of Drug-CD-Sepiolite (SV) Ternary Systems
2.5. Solid-State Characterization
2.6. Dissolution Rate Studies
2.7. Tablet Formulation and Characterization
3. Results and Discussion
3.1. Phase Solubility Studies
3.2. Characterization of the Drug–CD Binary Systems
3.3. Characterization of Drug–NC Binary Systems
3.4. Dissolution Rate Studies
3.5. Tablet Formulation and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Musini, V.M.; Nazer, M.; Bassett, K.; Wright, J.M. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst. Rev. 2014, 29, 003824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanphui, P.; Devi, V.K.; Clara, D.; Malviya, N.; Ganguly, S.; Desiraju, G.R. Cocrystals of hydrochlorothiazide: Solubility and diffusion/permeability enhancements through drug–coformer interactions. Mol. Pharm. 2015, 12, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.A.; Thaker, A.K.; Mohanraj, K. LC, LC-MS/MS studies for the identification and characterization of degradation products of hydrochlorothiazide and establishment of mechanistic approach towards degradation. J. Braz. Chem. Soc. 2012, 23, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Uekama, K.; Hirayama, F.; Tetsumi, I. Cyclodextrin drug carrier systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- Lovatti Alves, Q.; Barbosa Camargo, S.; Leonne Cruz de Jesus, R.; Flávia Silva, D. Drugs–β-Cyclodextrin inclusion complex: Would be a new strategy to improve Antihypertensive Therapy? Clin. Res. Trials 2019, 5, 1–3. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their therapeutic applications. Int. J. Pharm. 1998, 329, 1–11. [Google Scholar] [CrossRef]
- Mendes, C.; Buttchevitz, A.; Kruger, J.H.; Kratz, J.M.; Simões, C.M.; de Oliveira Benedet, P.; Oliveira, P.R.; Silva, M.A. “Inclusion complexes of hydrochlorothiazide and β-cyclodextrin: Physicochemical characteristics, in vitro and in vivo studies. Eur. J. Pharm. Sci. 2016, 83, 71–78. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Elzayat, E.M.; Alhowyan, A.A.; Alshehri, S.; Shakeel, F. Effect of β-cyclodextrin and different surfactants on solubility, stability, and permeability of hydrochlorothiazide. J. Mol. Liquids 2018, 250, 323–328. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Viseras, C.; Cerezo, P.; Sanchez, R.; Salcedo, I.; Aguzzi, C. Current challenges in clay minerals for drug delivery. Appl. Clay Sci. 2010, 48, 291–295. [Google Scholar] [CrossRef]
- Kinnari, P.; Mäkiläb, E.; Heikkiläb, T.; Salonen, J.; Hirvonen, J.; Santos, H.A. Comparison of mesoporous silicon and non-ordered mesoporous silica materials as drug carriers for itraconazole. Int. J. Pharm. 2011, 414, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Valleri, M.; Fabianelli, E.; Maestrelli, F.; Cirri, M. Characterization and evaluation of different mesoporous silica kinds as carriers for the development of effective oral dosage forms of glibenclamide. Int. J. Pharm. 2019, 563, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Leane, M.; Pitt, K.; Reynolds, G. The Manufacturing Classification System (MCS) Working Group. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.M.; Sousa Lobo, J.M. Cyclodextrins as excipients in tablet formulations. Drug Discov. Today 2018, 23, 1274–1284. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Aguzzi, C.; Viseras, C. Hybrid systems based on “drug—in cyclodextrin—in nanoclays” for improving oxaprozin dissolution properties. Int. J. Pharm. 2016, 509, 8–15. [Google Scholar] [CrossRef]
- Maestrelli, F.; Mura, P.; Cirri, M.; Mennini, N.; Ghelardini, C.; Di Cesare Mannelli, L. Development and characterization of fast dissolving tablets of oxaprozin based on hybrid systems of the drug with cyclodextrins and nanoclays. Int. J. Pharm. 2017, 531, 640–649. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Adv. Anal. Chem. Instr. 1965, 4, 117–212. [Google Scholar]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- Sareen, S.; Mathew, G.; Joseph, L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int. J. Pharm. Investig. 2012, 2, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, I.; Doshi, A.; Joshi, B.; Wankhede, V.; Doshi, J. Solid Dispersions: An approach to enhance solubility of poorly water soluble drug. Int. J. Sci. Innov. Res. 2013, 2, 685–694. [Google Scholar]
- Yendluri, R.; Otto, D.P.; De Villiers, M.M.; Vinokurovc, V.; Lvova, Y.M. Application of halloysite clay nanotubes as a pharmaceutical excipient. Int. J. Pharm. 2017, 521, 267–273. [Google Scholar] [CrossRef]
- Yendluri, R.; Lvov, Y.; De Villiers, M.M.; Vinokurov, V.; Naumenko, E.; Tarasova, E.; Fakhrullin, R. Paclitaxel Encapsulated in Halloysite Clay Nanotubes for Intestinal and Intracellular Delivery. J. Pharm. Sci. 2017, 106, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Lun, H.; Ouyang, J.; Yang, H. Natural halloysite nanotubes modified as aspirin carrier. RSC Adv. 2014, 83, 83. [Google Scholar] [CrossRef]
- Onnainty, R.; Shenfeld, E.M.; Quevedo, M.A.; Fernández, M.A.; Longhi, M.R.; Granero, G.E. Characterization of the Hydrochlorothiazide: β-cyclodextrin inclusion complex. Experimental and theoretical methods. J. Phys. Chem. B 2013, 117, 206–217. [Google Scholar] [CrossRef]
- Mennini, N.; Bragagni, M.; Maestrelli, F.; Mura, P. Physico-chemical characterization in solution and in the solid state of clonazepam complexes with native and chemically-modified cyclodextrins. J. Pharm. Biom. Anal. 2014, 89, 142–149. [Google Scholar] [CrossRef]
- Pires, M.A.; Souza dos Santos, R.A.S.; Sinisterra, R.D. Pharmaceutical Composition of Hydrochlorothiazide: β-Cyclodextrin: Preparation by Three Different Methods, Physico-Chemical Characterization and In Vivo Diuretic Activity Evaluation. Molecules 2011, 16, 4482–4499. [Google Scholar] [CrossRef] [Green Version]
- Hădărugă, D.I.; Birău (Mitroi), C.L.; Gruia, A.T.; Păunescu, V.; Geza, N.B.; Hădărugă, N.G. Moisture evaluation of β-cyclodextrin/fish oils complexes by thermal analyses: A data review on common barbel (Barbus barbus L.), Pontic shad (Alosa immaculata Bennett), European wels catfish (Silurus glanis L.), and common bleak (Alburnusalb L.) living in Danube river. Food Chem. 2017, 236, 49–58. [Google Scholar]
- Boldescu, V.; Bratu, I.; Borodi, G.; Kacso, I.; Bende, A.; Duca, G.; Macaev, F.; Pogrebnoi, S.; Ribkovskaia, Z. Study of binary systems of β -cyclodextrin with a highly potential anti-mycobacterial drug. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 129–135. [Google Scholar] [CrossRef]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Development and in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Viseras, C.; Aguzzi, C.; Cerezo, P. Medical and health applications of natural mineral nanotubes. In Natural Mineral Nanotubes; Apple Academic Press, Inc.: Palm Bay, FL, USA, 2015. [Google Scholar]
- Schipper, N.G.M.; Romeijn, S.G.; Verhoef, J.C.; Merkus, F.W.H.M. Nasal insulin delivery with dimethyl-β-cyclodextrin as an absorption enhancer in rabbits: Powder more effective than liquid formulations. Pharm. Res. 1993, 5, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.; Bizec, J.C.; Kis, G. Cyclodextrin derivatives and cyclofructan as ocular permeation enhancers. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 391–394. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]







| CD Type | K1:1 M−1 ± s.d. | CE | SE a |
|---|---|---|---|
| βCD | 131 ± 2 | 0.27 | 2.3 |
| HPβCD | 106 ± 3 | 0.28 | 3.1 |
| HEβCD | 114 ± 1 | 0.23 | 3.3 |
| SBEβCD | 213 ± 4 | 0.44 | 4.7 |
| RAMEB | 234 ± 2 | 0.48 | 4.8 |
| NC Type | HCT:NC Ratio (w/w) | HCT Melting Peak (°C) | HCT ΔHfus(J/g) | % RDC |
|---|---|---|---|---|
| ---- | / | 274.4 | 152.8 | 100.0 |
| PHC | 1:1 | 274.3 | 92.0 | 60.2 |
| PHC | 1:2 | 274.3 | 77.9 | 51.0 |
| PHC | 1:4 | 274.1 | 64.4 | 42.2 |
| VHS | 1:1 | 274.4 | 152.6 | 100.0 |
| VHS | 1:2 | 274.3 | 152.5 | 100.0 |
| VHS | 1:4 | 274.2 | 152.2 | 100.0 |
| SV | 1:1 | 274.4 | 84.2 | 55.1 |
| SV | 1:2 | 270.4 | 71.9 | 47.1 |
| SV | 1:4 | 269.2 | 56.5 | 37.0 |
| Batch | Drug Melting Peak (°C) | Hfus (J/g) | % RDC |
|---|---|---|---|
| HCT | 274.4 | 152.8 | 100.0 |
| PM | 269.2 | 56.5 | 37.0 |
| SS | 263.9 | 28.3 | 18.5 |
| SMS | 262.8 | 28.2 | 18.3 |
| GR | 260.4 | 15.4 | 10.1 |
| COF | 274.1 | 9.9 | 6.5 |
| SH | 274.0 | 8.2 | 5.4 |
| COE | / | / | / |
| Tablet | Drug Content (%) | Diameter (cm) | Thickness (cm) | Weight (mg) | Hardness (N) | Friability (%) | Disintegration Time (min) |
|---|---|---|---|---|---|---|---|
| Esidrex® | 98.5 ± 0.5 | 0.7 ± 0.0 | 0.25 ± 0.02 | 139.3 ± 1.9 | 4.0 ± 0.2 | 0.00 ± 0.00 | 6.0 ± 0.1 |
| New tablet | 99.2 ± 0.8 | 1.3 ± 0.0 | 0.20 ± 0.01 | 384.6 ± 0.5 | 6.6 ± 0.3 | 0.63 ± 0.01 | 13.0 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestrelli, F.; Cirri, M.; García-Villén, F.; Borrego-Sánchez, A.; Viseras Iborra, C.; Mura, P. Tablets of “Hydrochlorothiazide in Cyclodextrin in Nanoclay”: A New Nanohybrid System with Enhanced Dissolution Properties. Pharmaceutics 2020, 12, 104. https://doi.org/10.3390/pharmaceutics12020104
Maestrelli F, Cirri M, García-Villén F, Borrego-Sánchez A, Viseras Iborra C, Mura P. Tablets of “Hydrochlorothiazide in Cyclodextrin in Nanoclay”: A New Nanohybrid System with Enhanced Dissolution Properties. Pharmaceutics. 2020; 12(2):104. https://doi.org/10.3390/pharmaceutics12020104
Chicago/Turabian StyleMaestrelli, Francesca, Marzia Cirri, Fátima García-Villén, Ana Borrego-Sánchez, César Viseras Iborra, and Paola Mura. 2020. "Tablets of “Hydrochlorothiazide in Cyclodextrin in Nanoclay”: A New Nanohybrid System with Enhanced Dissolution Properties" Pharmaceutics 12, no. 2: 104. https://doi.org/10.3390/pharmaceutics12020104