Preparation of Co-Amorphous Systems by Freeze-Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Investigations
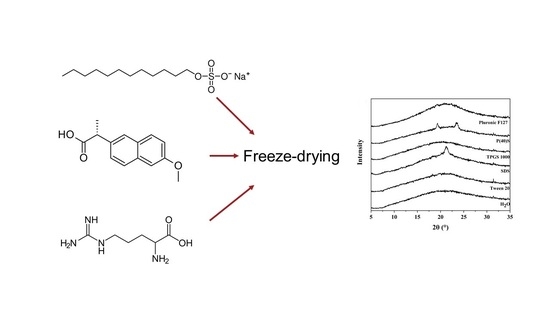
2.3. Freeze-Drying
2.4. X-Ray Powder Diffraction (XRPD)
2.5. Modulated Differential Scanning Calorimetry (mDSC) and Thermogravimetric Analysis (TGA)
2.6. Physical Stability
3. Results and Discussion
3.1. Solubility Investigations
3.2. Solid State Analysis of the Samples
3.3. Thermal Analysis of the Amorphous Samples
3.4. Stability Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chieng, N.; Aaltonen, J.; Saville, D.; Rades, T. Physical characterization and stability of amorphous indomethacin and ranitidine hydrochloride binary systems prepared by mechanical activation. Eur. J. Pharm. Biopharm. 2009, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Allesø, M.; Chieng, N.; Rehder, S.; Rantanen, J.; Rades, T.; Aaltonen, J. Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: Amorphous naproxen-cimetidine mixtures prepared by mechanical activation. J. Control. Release 2009, 136, 45–53. [Google Scholar] [CrossRef]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs–Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85, 873–881. [Google Scholar] [CrossRef]
- Löbmann, K.; Laitinen, R.; Strachan, C.; Rades, T.; Grohganz, H. Amino acids as co-amorphous stabilizers for poorly water-soluble drugs–Part 2: Molecular interactions. Eur. J. Pharm. Biopharm. 2013, 85, 882–888. [Google Scholar] [CrossRef]
- Wu, W.; Ueda, H.; Löbmann, K.; Rades, T.; Grohganz, H. Organic acids as co-formers for co-amorphous systems–Influence of variation in molar ration on the physicochemical properties of the co-amorphous systems. Eur. J. Pharm. Biopharm. 2018, 131, 25–32. [Google Scholar] [CrossRef]
- Fung, M.; Bērziņš, K.; Suryanarayanan, R. Physical Stability and Dissolution Behavior of Ketoconazole-Organic Acid Coamorphous Systems. Mol. Pharm. 2018, 15, 1862–1869. [Google Scholar] [CrossRef]
- Gao, Y.; Liao, J.; Qi, X.; Zhang, J. Coamorphous repaglinide-saccharin with enhanced dissolution. Int. J. Pharm. 2013, 450, 290–295. [Google Scholar] [CrossRef]
- Qian, S.; Heng, W.; Wei, Y.; Zhang, J.; Gao, Y. Coamorphous Lurasidone Hydrochloride-Saccharin with Charge-Assisted Hydrogen Bonding Interaction Shows Improved Physical Stability and Enhanced Dissolution with pH-Independent Solubility Behavior. Cryst. Growth Des. 2015, 15, 2920–2928. [Google Scholar] [CrossRef]
- Shayanfar, A.; Ghavimi, H.; Hamishehkar, H.; Jouyban, A. Coamorphous Atorvastatin Calcium to Improve its Physicochemical and Pharmacokinetic Properties. J. Pharm. Pharm. Sci. 2013, 16, 577–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.M.A.; Al-Remawi, M.M.A. Freeze Dried Quetiapine-Nicotinamide Binary Solid Dispersions: A New Strategy for Improving Physicochemical Properties and Ex Vivo Diffusion. J. Pharm. 2016, 2016, 2126056. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Löbmann, K.; Grohganz, H.; Rades, T. Co-former selection for co-amorphous drug-amino acid formulations. Int. J. Pharm. 2019, 557, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Rades, T.; Löbmann, K.; Grohganz, H. Influence of Solvent Composition on the Performance of Spray-Dried Co-Amorphous Formulations. Pharmaceutics 2018, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasten, G.; Duarte, I.; Paisana, M.; Löbmann, K.; Rades, T.; Grohganz, H. Process Optimization and Upscaling of Spray-Dried Drug-Amino acid Co-Amorphous Formulations. Pharmaceutics 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saß, A.; Lee, G. Evaluation of some water-miscible organic solvents for spray-drying enzymes and carbohydrates. Drug Dev. Ind. Pharm. 2014, 40, 749–757. [Google Scholar] [CrossRef]
- Pisano, R.; Fissore, D.; Barresi, A.A.; Rastelli, M. Quality by Design: Scale-Up of Freeze-Drying Cycles in Pharmaceutical Industry. AAPS PharmSciTech. 2013, 14, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- Roy, I.; Gupta, M.N. Freeze-drying of proteins: Some emerging concerns. Biotechnol. Appl. Biochem. 2004, 39, 165–177. [Google Scholar] [CrossRef]
- Shamblin, S.L.; Taylor, L.S.; Zografi, G. Mixing Behavior of Colyophilized Binary Systems. J. Pharm. Sci. 1998, 87, 694–701. [Google Scholar] [CrossRef]
- Izutsu, K.; Fujimaki, Y.; Kuwabara, A.; Aoyagi, N. Effect of counterions on the physical properties of L-arginine in frozen solutions and freeze-dried solids. Int. J. Pharm. 2005, 301, 161–169. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, H.; Babu, S.; Garad, S. Co-Amorphous Formation of High-Dose Zwitterionic Compounds with Amino Acids To Improve Solubility and Enable Parenteral Delivery. Mol. Pharm. 2018, 15, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Schuldt-Lieb, S.; Gieseler, H. Freeze-Drying From Organic Cosolvent Systems, Part 1: Thermal Analysis of Cosolvent-Based Placebo Formulations in the Frozen State. J. Pharm. Sci. 2017, 107, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Teagarden, D.L.; Baker, D.S. Practical aspects of lyophilization using non-aqueous co-solvent systems. Eur. J. Pharm. Sci. 2001, 15, 115–133. [Google Scholar] [CrossRef]
- Daoussi, R.; Bogdani, E.; Vessot, S.; Andrieu, J.; Monnier, O. Freeze-Drying of an Active Principle Ingredient Using Organic Co-Solvent Formulations: Influence of Freezing Conditions and Formulation on Solvent Crystals Morphology, Thermodynamics Data, and Sublimation Kinetics. Dry. Technol. 2011, 29, 1858–1867. [Google Scholar] [CrossRef]
- Mah, P.T.; Peltonen, L.; Novakovic, D.; Rades, T.; Strachan, C.J.; Laaksonen, T. The effect of surfactants on the dissolution behavior of amorphous formulations. Eur. J. Pharm. Biopharm. 2016, 103, 13–22. [Google Scholar] [CrossRef]
- Kasten, G.; Lobo, L.; Dengale, S.; Grohganz, H.; Rades, T.; Löbmann, K. In vitro and in vivo comparison between crystalline and co-amorphous salts of naproxen-arginine. Eur. J. Pharm. Biopharm. 2018, 132, 192–199. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of Diluent and of Copolymer Composition on the Glass Temperature of a Polymer System. Bull. Amer. Phys. Soc. 1956, 1, 123. [Google Scholar]




| System | NAP-ARG | NAP-LYS | SDS | T20 | TPGS | P (40) S | PF127 |
|---|---|---|---|---|---|---|---|
| Tg (°C) | 65 | 59 | −9.1 | <−100 | −24.1 | −6.6 | −66.5 |
| Experimental | Calculated | ||||||
|---|---|---|---|---|---|---|---|
| Surfactant System | Tg 1 (°C) | Tg 2 (°C) | Water Content (w %) | Tg 1 (°C) | Tg 2 (°C) | Theoretical Tg of Homogenous System | Duration until Recrystallization (weeks) |
| H2O-med | 60 | 3.5 | 67 | 8 | |||
| H2O-high | 64 | 3.1 | 70 | 8 | |||
| SDS-low | 38 | 2.0 | 42 | 41 | 4 | ||
| SDS-med | 39 | 1.0 | 40 | 30 | 4 | ||
| SDS-high | 36 | 0.9 | 38 | 30 | 4 | ||
| P40S-low | 27 | 2.1 | 31 | 41 | >18 | ||
| P40S-med | 25 | 1.4 | 27 | 31 | >18 | ||
| P40S-high | 28 | 0.3 | 28 | 31 | >18 | ||
| PF127-low | 41 | 0.5 | 42 | 25 | >18 | ||
| PF127-med | 36 | 0.5 | 37 | 6 | >18 | ||
| PF127-high | 37 | 0.8 | 38 | 6 | >18 | ||
| T20-low | n.d. | 1.3 | n.d. | 16 * | >18 | ||
| T20-med | n.d. | 1.2 | n.d. | −8 * | >18 | ||
| T20-high | n.d. | 0.8 | n.d. | −8 * | >18 | ||
| TPGS-low | 27 | 34 | 1.5 | 30 | 37 | 37 | >18 |
| TPGS-med | 29 | 34 | 0.8 | 30 | 36 | 24 | >18 |
| TPGS-high | 29 | 33 | 0.9 | 31 | 35 | 24 | >18 |
| Experimental | Calculated | ||||||
|---|---|---|---|---|---|---|---|
| Surfactant System | Tg 1 (°C) | Tg 2 (°C) | Water Content (w %) | Tg 1 (°C) | Tg 2 (°C) | Theoretical Tg of Homogenous System | Duration until Recrystallization (weeks) |
| H2O-med | 65 | 76 | 5.0 | 76 | 87 | 4 | |
| H2O-high | 60 | 81 | 2.2 | 64 | 86 | 8 | |
| T20-low | 33 | 67 | 2.5 | 37 | 72 | 23 * | 18 |
| T20-med | 43 | 68 | 1.2 | 45 | 71 | −2 * | 4 |
| T20-high | 46 | 69 | 1.3 | 48 | 72 | −2 * | 4 |
| TPGS-low | 32 | 58 | 2.3 | 36 | 63 | 42 | 8 |
| TPGS-med | 33 | 58 | 1.7 | 36 | 61 | 29 | 18 |
| TPGS-high | 34 | 57 | 1.4 | 37 | 60 | 29 | 8 |
| SDS-low | 38 | 65 | 2.0 | 42 | 70 | 46 | >18 |
| SDS-med | 33 | 66 | 1.5 | 36 | 69 | 35 | >18 |
| SDS-high | 42 | 65 | 1.6 | 44 | 68 | 35 | >18 |
| P40S-low | 14 | 2.2 | 18 | 47 | 18 | ||
| P40S-med | 27 | 2.1 | 31 | 36 | >18 | ||
| P40S-high | 38 | 2.0 | 41 | 36 | 18 | ||
| PF127-low | 23 | 1.1 | 24 | 32 | 8 | ||
| PF127-med | 16 | 0.7 | 17 | 12 | 8 | ||
| PF127-high | 24 | 1.9 | 27 | 12 | 8 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wostry, M.; Plappert, H.; Grohganz, H. Preparation of Co-Amorphous Systems by Freeze-Drying. Pharmaceutics 2020, 12, 941. https://doi.org/10.3390/pharmaceutics12100941
Wostry M, Plappert H, Grohganz H. Preparation of Co-Amorphous Systems by Freeze-Drying. Pharmaceutics. 2020; 12(10):941. https://doi.org/10.3390/pharmaceutics12100941
Chicago/Turabian StyleWostry, Melvin, Hanna Plappert, and Holger Grohganz. 2020. "Preparation of Co-Amorphous Systems by Freeze-Drying" Pharmaceutics 12, no. 10: 941. https://doi.org/10.3390/pharmaceutics12100941