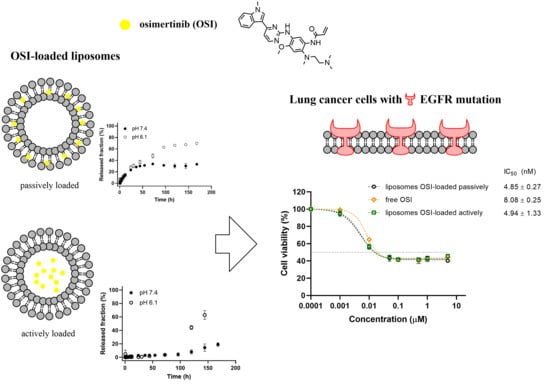
Development of Liposomal Vesicles for Osimertinib Delivery to EGFR Mutation—Positive Lung Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Liposomes
2.2.2. Dynamic Light Scattering (DLS)
2.2.3. Stability of Liposomes
2.2.4. In Vitro Release of OSI from Liposomes
2.2.5. Cell Culture
2.2.6. In Vitro Cellular Cytotoxicity
2.2.7. Cellular Uptake and Localization of Liposomes
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation Method and Composition
3.2. Encapsulation Efficacy
3.3. Liposomes Size
3.4. Stability
3.5. In Vitro Drug Release
3.6. Mathematical Modeling of OSI Release from Liposomes
3.7. Cellular Uptake and Localization of Liposomes
3.8. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Cancer Statistics-Eurostat. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cancer_statistics_-_specific_cancers#Lung_cancer (accessed on 5 May 2020).
- Zeng, X.; Wan, X.; Xu, J.; Wang, H.; Chen, H.; Zeng, Q.; Zhang, W.; Zhao, B. Therapeutic options for advanced epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer: A Bayesian network secondary analysis. Aging 2020, 12, 7129. [Google Scholar] [CrossRef] [PubMed]
- Halliday, P.R.; Blakely, C.M.; Bivona, T.G. Emerging targeted therapies for the treatment of non-small cell lung cancer. Curr. Oncol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Politi, K.; Herbst, R.S. Lung cancer in the era of precision medicine. Clin. Cancer Res. 2015, 21, 2213–2220. [Google Scholar] [CrossRef] [Green Version]
- Pao, W.; Chmielecki, J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat. Rev. Cancer 2010, 10, 760–774. [Google Scholar] [CrossRef] [Green Version]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Zhou, C.; Hu, C.-P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR TKI therapy in 155 patients with EGFR mutant lung cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingam, S.S.; Gray, J.E.; Ohe, Y.; Cho, B.C.; Vansteenkiste, J.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; Shah, R.; et al. LBA5_PR-Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): Final overall survival analysis. Ann. Oncol. 2019, 30, v914–v915. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Tagrisso. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tagrisso (accessed on 5 May 2020).
- Vrignaud, S.; Hureaux, J.; Wack, S.; Benoit, J.-P.; Saulnier, P. Design, optimization and in vitro evaluation of reverse micelle-loaded lipid nanocarriers containing erlotinib hydrochloride. Int. J. Pharm. 2012, 436, 194–200. [Google Scholar] [CrossRef]
- Trummer, B.J.; Iyer, V.; Balu-Iyer, S.V.; O’Connor, R.; Straubinger, R.M. Physicochemical properties of EGF receptor inhibitors and development of a nanoliposomal formulation of gefitinib. J. Pharm. Sci. 2012, 101, 2763–2776. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Tao, H.; Shi, K.-H. Development of a nanoliposomal formulation of erlotinib for lung cancer and in vitro/in vivo antitumoral evaluation. Drug Des. Dev. Ther. 2017, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; He, C.; Liu, Y.; Jiang, J.; Ma, T. Novel therapeutic modalities and drug delivery–erlotinib liposomes modified with galactosylated lipid: In vitro and in vivo investigations. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1902–1907. [Google Scholar] [CrossRef] [Green Version]
- Lakkadwala, S.; dos Santos Rodrigues, B.; Sun, C.; Singh, J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J. Control. Release 2019, 307, 247–260. [Google Scholar] [CrossRef]
- Lu, X.; Liu, S.; Han, M.; Yang, X.; Sun, K.; Wang, H.; Mu, H.; Du, Y.; Wang, A.; Ni, L.; et al. Afatinib-loaded immunoliposomes functionalized with cetuximab: A novel strategy targeting the epidermal growth factor receptor for treatment of non-small-cell lung cancer. Int. J. Pharm. 2019, 560, 126–135. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kuzmov, A.; Minko, T. Nanotechnology approaches for inhalation treatment of lung diseases. J. Control. Release 2015, 219, 500–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbuzenko, O.B.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics 2019, 9, 8362–8376. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Saad, M.; Pozharov, V.P.; Reuhl, K.R.; Mainelis, G.; Minko, T. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc. Natl. Acad. Sci. USA 2010, 107, 10737–10742. [Google Scholar] [CrossRef] [Green Version]
- Garbuzenko, O.B.; Mainelis, G.; Taratula, O.; Minko, T. Inhalation treatment of lung cancer: The influence of composition, size and shape of nanocarriers on their lung accumulation and retention. Cancer Biol. Med. 2014, 11, 44–55. [Google Scholar] [CrossRef]
- Zang, X.; Lee, J.B.; Deshpande, K.; Garbuzenko, O.B.; Minko, T.; Kagan, L. Prevention of paclitaxel-induced neuropathy by formulation approach. J. Control. Release 2019, 303, 109–116. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Han, J.-Y.; Katakami, N.; Kim, H.R.; Hodge, R.; Kaur, P.; Brown, A.P.; Ghiorghiu, D.; et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: Data from a randomized phase III trial (AURA3). J. Clin. Oncol. 2018, 36, 2702–2709. [Google Scholar] [CrossRef]
- Vishwanathan, K.; Varrone, A.; Varnas, K.; Jucaite, A.; Cselenyi, Z.; Johnstrom, P.; Schou, M.; Vasquez-Romero, A.; Moein, M.M.; Halldin, C.; et al. Abstract CT013: Osimertinib displays high brain exposure in healthy subjects with intact blood-brain barrier: A microdose positron emission tomography (PET) study with 11C-labelled osimertinib. Cancer Res. 2018, 78, CT013. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.L.; Han, J.; Park, S.; Jung, H.A.; Su, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; Ahn, M.-J. Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J. Thorac. Oncol. 2020. [Google Scholar] [CrossRef]
- Yang, J.C.H.; Kim, S.-W.; Kim, D.-W.; Lee, J.-S.; Cho, B.C.; Ahn, J.-S.; Lee, D.H.; Kim, T.M.; Goldman, J.W.; Natale, R.B.; et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: The BLOOM study. J. Clin. Oncol. 2020, 38, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almurshedi, A.S.; Radwan, M.; Omar, S.; Alaiya, A.A.; Badran, M.M.; Elsaghire, H.; Saleem, I.Y.; Hutcheon, G.A. A novel pH-sensitive liposome to trigger delivery of afatinib to cancer cells: Impact on lung cancer therapy. J. Mol. Liq. 2018, 259, 154–166. [Google Scholar] [CrossRef]
- Lei, M.; Ma, G.; Sha, S.; Wang, X.; Feng, H.; Zhu, Y.; Du, X. Dual-functionalized liposome by co-delivery of paclitaxel with sorafenib for synergistic antitumor efficacy and reversion of multidrug resistance. Drug Deliv. 2019, 26, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Gabizon, A.; Martin, F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 1997, 54 (Suppl. 4), 15–21. [Google Scholar] [CrossRef]
- Haran, G.; Cohen, R.; Bar, L.K.; Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta 1993, 1151, 201–215. [Google Scholar] [CrossRef]
- Barenholz, Y. (Chezy) Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Naziris, N.; Pippa, N.; Demetzos, C. The significance of drug-to-lipid ratio to the development of optimized liposomal formulation. J. Liposome Res. 2018, 28, 249–258. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Liposomes and the Use of Zeta Potential Measurements to Study Sterically Stabilized Liposomes. Available online: https://www.azonano.com/article.aspx?ArticleID=1214 (accessed on 12 June 2020).
- Li, T.; Cipolla, D.; Rades, T.; Boyd, B.J. Drug nanocrystallisation within liposomes. J. Control. Release 2018, 288, 96–110. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Maurer, N.; Akhong, Q.-F.; Leone, R.; Leng, E.; Wang, J.; Semple, S.C.; Cullis, P.R. Liposome-encapsulated vincristine, vinblastine and vinorelbine: A comparative study of drug loading and retention. J. Control. Release 2005, 104, 103–111. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoshino, K.; Yamashita, K.; Kasukawa, H. Designing a novel in vitro drug-release-testing method for liposomes prepared by pH-gradient method. Int. J. Pharm. 2012, 430, 381–387. [Google Scholar] [CrossRef]
- Bruschi, M.L. 5-Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Levchenko, T.S.; Whiteman, K.R.; Yaroslavov, A.A.; Tsatsakis, A.M.; Rizos, A.K.; Michailova, E.V.; Shtilman, M.I. Amphiphilic poly-N-vinylpyrrolidones: Synthesis, properties and liposome surface modification. Biomaterials 2001, 22, 3035–3044. [Google Scholar] [CrossRef]
- Liu, X.; Huang, G. Formation strategies, mechanism of intracellular delivery and potential clinical applications of pH-sensitive liposomes. Asian J. Pharm. Sci. 2013, 8, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Koshkaryev, A.; Thekkedath, R.; Pagano, C.; Meerovich, I.; Torchilin, V.P. Targeting of lysosomes by liposomes modified with octadecyl-rhodamine B. J. Drug Target. 2011, 19, 606–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahay, G.; Kim, J.O.; Kabanov, A.V.; Bronich, T.K. The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents. Biomaterials 2010, 31, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, L.; Meng, X.; Wang, J.; Zhu, L.; Liu, C.; Li, S.; Zheng, L.; Yang, Z.; Xing, L.; et al. Osimertinib (AZD9291) increases radio-sensitivity in EGFR T790M non-small cell lung cancer. Oncol. Rep. 2019, 41, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.J.; Eberlein, C.; Taylor, M.; Ashton, S.; Robinson, D.; Cross, D. Inhibition of oxidative phosphorylation suppresses the development of osimertinib resistance in a preclinical model of EGFR-driven lung adenocarcinoma. Oncotarget 2016, 7, 86313–86325. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, D.; Takahashi, F.; Mitsuishi, Y.; Tajima, K.; Hidayat, M.; Winardi, W.; Ihara, H.; Kanamori, K.; Matsumoto, N.; Asao, T.; et al. Activation of insulin-like growth factor-1 receptor confers acquired resistance to osimertinib in non-small cell lung cancer with EGFR T790M mutation. Thorac. Cancer 2020, 11, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Gao, F.; Li, W.; Zhou, L.; Liu, W.; Li, M. Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2020, 39, 62. [Google Scholar] [CrossRef] [Green Version]
- van Hoogevest, P. Review—An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef]







| Formulation | Liposome Components | Initial Molar Ratio | Size (nm) | PDI | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|---|---|
| Thin Lipid Hydration—Passive Loading | ||||||
| 1−NL | Egg−PC/chol/PEG2000−DSPE | 55/40/5 | 108.7 ± 1.3 | 0.101 | −10.9 ± 0.3 | |
| 1−OSI | Egg−PC/chol/PEG2000−DSPE/OSI | 55/40/5/7 | 114.8 ± 0.8 | 0.043 | −11.4 ± 0.8 | 81.5 ± 6.6 |
| 2−NL | DPPC/chol/PEG2000−DSPE | 55/40/5 | 122.0 ± 1.0 | 0.036 | −11.5 ± 0.0 | |
| 2−OSI | DPPC/chol/PEG2000−DSPE/OSI | 55/40/5/7 | 119.8 ± 1.4 | 0.047 | −10.3 ± 0.7 | 54.2 ±10.8 |
| 3−NL | DSPC/chol/PEG2000−DSPE | 55/40/5 | 124.4 ± 2.0 | 0.049 | −11.6 ± 1.4 | |
| 3−OSI | DSPC/chol/PEG2000−DSPE/OSI | 55/40/5/7 | 125.7 ± 0.6 | 0.016 | −10.4 ± 0.5 | 48.6 ± 7.6 |
| Thin Lipid Hydration—Ammonium Sulfate Gradient−Assisted Loading | ||||||
| 4−NL | Egg−PC/chol/PEG2000−DSPE | 55/40/5 | 110.9 ± 1.0 | 0.042 | −10.3 ± 0.2 | |
| 4−OSI | Egg−PC/chol/PEG2000−DSPE/OSI | 55/40/5/7 | 109.6 ± 0.1 | 0.051 | −10.2 ± 0.9 | 97.2 ± 0.7 |
| 5−NL | DPPC/chol/PEG2000−DSPE | 55/40/5 | 124.0 ± 0.6 | 0.030 | −10.2 ± 0.9 | |
| 5−OSI | DPPC/chol/PEG2000−DSPE/OSI | 55/40/5/7 | 119.1 ± 1.4 | 0.047 | −11.9 ± 1.5 | 92.6 ± 3.6 |
| 6−NL | DSPC/chol/PEG2000−DSPE | 55/40/5 | 125.5 ± 0.6 | 0.097 | −11.5 ± 1.1 | |
| 6−OSI | DSPC/chol/PEG2000−DSPE/OSI | 55/40/5/7 | 122.7 ± 1.0 | 0.025 | −11.3 ± 1.3 | 94.2 ± 5.6 |
| Model | Parameter | Formulation | |||||
|---|---|---|---|---|---|---|---|
| 1−OSI | 2−OSI | 3−OSI | 1−OSI | 2−OSI | 3−OSI | ||
| pH 7.4 | pH 6.1 | ||||||
| Higuchi; | |||||||
| kH | 3.365 | 5.816 | 10.179 | 5.595 | 8.141 | 11.423 | |
| R2 | 0.805 | 0.882 | 0.963 | 0.955 | 0.942 | 0.953 | |
| AIC | 116.350 | 126.699 | 57.458 | 71.072 | 61.275 | 53.025 | |
| T50 (h) | 220.772 | 73.910 | 24.130 | 79.865 | 37.723 | 19.161 | |
| Korsmeyer−Peppas; | |||||||
| kKP | 6.767 | 10.490 | 7.805 | 2.921 | 5.126 | 8.030 | |
| n | 0.342 | 0.367 | 0.593 | 0.665 | 0.650 | 0.644 | |
| R2 | 0.886 | 0.935 | 0.978 | 0.990 | 0.980 | 0.982 | |
| AIC | 108.714 | 117.946 | 53.791 | 53.748 | 51.578 | 45.218 | |
| T50 (h) | 346.892 | 70.771 | 22.899 | 71.364 | 33.181 | 17.129 | |
| Peppas−Sahlin; | |||||||
| k1 | 3.664 | 6.200 | 5.244 | 2.313 | −21.099 | −33.843 | |
| k2 | −0.095 | −0.157 | −0.111 | 1.209 | 22.926 | 38.891 | |
| m | 0.641 | 0.624 | 0.865 | 0.401 | 0.200 | 0.176 | |
| R2 | 0.974 | 0.985 | 0.989 | 0.988 | 0.990 | 0.994 | |
| AIC | 82.091 | 91.581 | 45.395 | 55.059 | 42.983 | 33.540 | |
| T50 (h) | None Calc | 48.988 | 19.872 | 71.670 | 32.513 | 17.074 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skupin-Mrugalska, P.; Minko, T. Development of Liposomal Vesicles for Osimertinib Delivery to EGFR Mutation—Positive Lung Cancer Cells. Pharmaceutics 2020, 12, 939. https://doi.org/10.3390/pharmaceutics12100939
Skupin-Mrugalska P, Minko T. Development of Liposomal Vesicles for Osimertinib Delivery to EGFR Mutation—Positive Lung Cancer Cells. Pharmaceutics. 2020; 12(10):939. https://doi.org/10.3390/pharmaceutics12100939
Chicago/Turabian StyleSkupin-Mrugalska, Paulina, and Tamara Minko. 2020. "Development of Liposomal Vesicles for Osimertinib Delivery to EGFR Mutation—Positive Lung Cancer Cells" Pharmaceutics 12, no. 10: 939. https://doi.org/10.3390/pharmaceutics12100939