Rapid Preparation of Spherical Granules via the Melt Centrifugal Atomization Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spherical Granules
2.3. Characterization of Granules
2.4. Thermal Analysis
2.5. Melt Rheological Study
2.6. X-ray Diffraction (XRD) Analysis
2.7. In Vitro Dissolution Study
3. Results and Discussion
3.1. Influence of Drugs and Excipients on Granule Formability
3.2. Influence of Operating Parameters on the Morphology and Yield of Granules
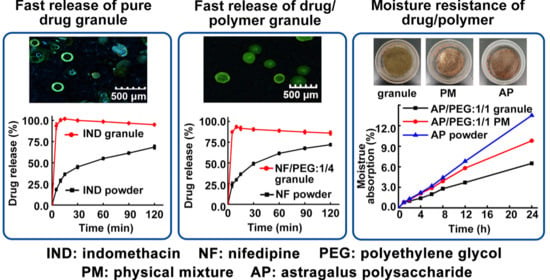
3.3. Moisture Absorption of Granules
3.4. Immediate Release Characteristics of Granules
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| MCA | melt centrifugal atomization |
| IND | indomethacin |
| NF | nifedipine |
| TNZ | tinidazole |
| MT | metoprolol tartrate |
| AP | astragalus polysaccharide |
| PEG | polyethylene glycol |
| SOL | Soluplus® |
| SA | stearic acid |
| GMDS | glycerol monostearate/distearate |
| RL | Eudragit® RL PO |
| TGA | thermal gravimetric analysis |
| Td | thermal decomposition temperature |
| DSC | differential scanning calorimetry |
| Tm | melting temperature |
| η* | complex viscosity |
| XRD | X-ray diffraction |
| PM | physical mixture |
References
- Cavallari, C.; Gonzalez-Rodriguez, M.; Tarterini, F.; Fini, A. Image analysis of lutrol/gelucire/olanzapine microspheres prepared by ultrasound-assisted spray congealing. Eur. J. Pharm. Biopharm. 2014, 88, 909–918. [Google Scholar] [CrossRef]
- Song, Y.; Zemlyanov, D.; Chen, X.; Su, Z.; Nie, H.; Lubach, J.W.; Smith, D.; Byrn, S.; Pinal, R. Acid-base interactions in amorphous solid dispersions of lumefantrine prepared by spray-drying and hot-melt extrusion using X-ray photoelectron spectroscopy. Int. J. Pharm. 2016, 514, 456–464. [Google Scholar] [CrossRef]
- Shiino, K.; Iwao, Y.; Fujinami, Y.; Itai, S. Preparation and evaluation of granules with ph-dependent release by melt granulation. Int. J. Pharm. 2012, 431, 70–77. [Google Scholar] [CrossRef]
- Walsh, D.; Serrano, D.R.; Worku, Z.A.; Madi, A.M.; O‘Connell, P.; Twamley, B.; Healy, A.M. Engineering of pharmaceutical cocrystals in an excipient matrix: Spray drying versus hot melt extrusion. Int. J. Pharm. 2018, 551, 241–256. [Google Scholar] [CrossRef]
- Oh, C.M.; Heng, P.W.S.; Chan, L.W. Influence of Hydroxypropyl Methylcellulose on Metronidazole Crystallinity in Spray-Congealed Polyethylene GlycolMicroparticles and Its Impact withVarious Additives on Metronidazole Release. AAPS PharmSciTech 2015, 6, 1357–1366. [Google Scholar] [CrossRef]
- Ouyang, H.; Zheng, A.; Heng, P.; Chan, L. Effect of Lipid Additives and Drug on the Rheological Properties of Molten Paraffin Wax, Degree of Surface Drug Coating, and Drug Release in Spray-Congealed Microparticles. Pharmaceutics 2018, 10, 75. [Google Scholar] [CrossRef]
- Tian, L.; Anderson, I.; Riedemann, T.; Russell, A. Production of fine calcium powders by centrifugal atomization with rotating quench bath. Powder Technol. 2017, 308, 84–93. [Google Scholar] [CrossRef]
- Akio, F.; Shinnosuke, A.; Kazuki, S.; Yamato, H.; Hirotsugu, T. Synthesis of Lead-Free Solder Particles Using High-Speed Centrifugal Atomization. Mater. Trans. 2017, 58, 1458–1462. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Zhou, Y.; Shan, W.; Shen, J. Mechanistic study on rapid fabrication of fibrous films via centrifugal melt spinning. Int. J. Pharm. 2019, 560, 155–165. [Google Scholar] [CrossRef]
- Goh, H.P.; Heng, P.W.S.; Liew, C. Comparative evaluation of powder flow parameters with reference to particle size and shape. Int. J. Pharm. 2018, 547, 133–141. [Google Scholar] [CrossRef]
- Khan, G.; Patel, R.R.; Yadav, S.K.; Kumar, N.; Chaurasia, S.; Ajmal, G.; Mishra, P.K.; Mishra, B. Development, optimization and evaluation of tinidazole functionalized electrospun poly(ε-caprolactone) nanofiber membranes for the treatment of periodontitis. RSC Adv. 2016, 6, 100214–100229. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef]
- Maru, S.M.; de Matas, M.; Kelly, A.; Paradkar, A. Characterization of thermal and rheological properties of zidovidine, lamivudine and plasticizer blends with ethyl cellulose to assess their suitability for hot melt extrusion. Eur. J. Pharm. Sci. 2011, 44, 471–478. [Google Scholar] [CrossRef]
- Shimada, Y.; Komaki, H.; Hirai, A.; Goto, S.; Hashimoto, Y.; Uchiro, H.; Terada, H. Decarboxylation of indomethacin induced by heat treatment. Int. J. Pharm. 2018, 545, 51–56. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Ma, G.; Sun, J.; Zhang, Y.; Jing, Y.; Jia, Y. A novel low-temperature phase change material based on stearic acid and hexanamide eutectic mixture for thermal energy storage. Chem. Phys. Lett. 2019, 714, 166–171. [Google Scholar] [CrossRef]
- Wu, C.; van de Weert, M.; Baldursdottir, S.G.; Yang, M.; Mu, H. Effect of excipients on encapsulation and release of insulin from spray-dried solid lipid microparticles. Int. J. Pharm. 2018, 550, 439–446. [Google Scholar] [CrossRef]
- Zardini, A.A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef]
- Marano, S.; Barker, S.A.; Raimi-Abraham, B.T.; Missaghi, S.; Rajabi-Siahboomi, A.; Craig, D.Q.M. Development of micro-fibrous solid dispersions of poorly water-soluble drugs in sucrose using temperature-controlled centrifugal spinning. Eur. J. Pharm. Biopharm. 2016, 103, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Piscitelli, F.; Saccone, G.; Gianvito, A.; Cosentino, G.; Mazzola, L. Characterization and manufacturing of a paraffin wax as fuel for hybrid rockets. Propul. Power Res. 2018, 7, 218–230. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Luo, Y.; Yao, Q.; Zhong, Y.; Tian, B.; Tang, X. Extruded Soluplus/SIM as an oral delivery system: Characterization, interactions, in vitro and in vivo evaluations. Drug Deliv. 2016, 23, 1902–1911. [Google Scholar] [CrossRef]
- Quinten, T.; Andrews, G.P.; De Beer, T.; Saerens, L.; Bouquet, W.; Jones, D.S.; Hornsby, P.; Remon, J.P.; Vervaet, C. Preparation and Evaluation of Sustained-Release Matrix Tablets Based on Metoprolol and an Acrylic Carrier Using Injection Moulding. AAPS PharmSciTech 2012, 13, 1197–1211. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Russell, A. Phase field study of interfacial diffusion-driven spheroidization in a composite comprised of two mutually insoluble phases. J. Chem. Phys. 2014, 140, 124706. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Zhu, X.; Liao, Q.; Ding, B. Centrifugal granulation performance of liquid with various viscosities for heat recovery of blast furnace slag. Appl. Therm. Eng. 2015, 89, 494–504. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Li, X.; Liu, C.; Yang, B. A comparative study of jet formation in nozzle- and nozzle-less centrifugal spinning systems. J. Polym. Sci. Part B 2014, 52, 1547–1559. [Google Scholar] [CrossRef]










| Drugs | Tm (°C) | Td (°C) | Hydrophilic Excipients | Tm (°C) | Td (°C) | Hydrophobic Excipients | Tm (°C) | Td (°C) |
|---|---|---|---|---|---|---|---|---|
| IND | 162 | 220 | PEG [15] | 60 | 290 | SA [16] | 60 | 250 |
| NF | 174 | 215 | mannitol [4] | 166 | 270 | GMDS [17,18] | 56 | 125 |
| TNZ | 124 | 220 | Sucrose [19] | 185 | 210 | paraffin [6,20] | 61 | 235 |
| MT | 123 | 180 | SOL [21] | / | 250 | RL [22] | / | 170 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zheng, N.; Wang, X.; Ivone, R.; Shan, W.; Shen, J. Rapid Preparation of Spherical Granules via the Melt Centrifugal Atomization Technique. Pharmaceutics 2019, 11, 198. https://doi.org/10.3390/pharmaceutics11050198
Yang Y, Zheng N, Wang X, Ivone R, Shan W, Shen J. Rapid Preparation of Spherical Granules via the Melt Centrifugal Atomization Technique. Pharmaceutics. 2019; 11(5):198. https://doi.org/10.3390/pharmaceutics11050198
Chicago/Turabian StyleYang, Yan, Nan Zheng, Xiaoyue Wang, Ryan Ivone, Weiguang Shan, and Jie Shen. 2019. "Rapid Preparation of Spherical Granules via the Melt Centrifugal Atomization Technique" Pharmaceutics 11, no. 5: 198. https://doi.org/10.3390/pharmaceutics11050198