Novel Universal Recombinant Rotavirus A Vaccine Candidate: Evaluation of Immunological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Rotavirus Recombinant Antigen
2.2. Obtaining Sera to Untyped Field Rotavirus Isolate
2.3. Western Blot Analysis
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) for Qualitative Assessment of Protein-Serum Interaction
2.5. TMV Isolation and Spherical Particles Generation
2.6. Immunofluorescence Analysis
2.7. Immunisation of Mice for the Immunogenicity Studies
2.8. Ethical Statement
2.9. Statistical Analysis
2.10. Enzyme-Linked Immunosorbent Assay (ELISA) for Titre Measurement
3. Results
3.1. Designing Universal Recombinant Rotavirus Antigen URRA
3.2. Interaction of URRA with Antisera to Patient-Derived Field Rotavirus Isolates
3.3. The Adsorption of URRA to SPs
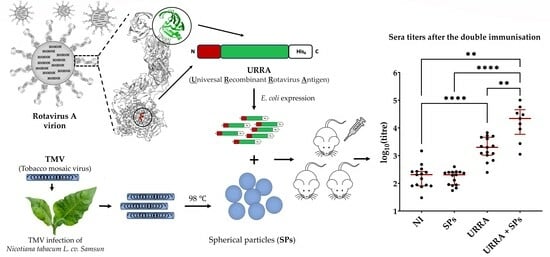
3.4. The Immunogenicity of Individual URRA and of a Vaccine Candidate (URRA + SPs)
4. Discussion
5. Conclusions
6. Limitations of the Current Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Kang, G. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef]
- Varghese, T.; Gagandeep, K.; Steele, A. Understanding Rotavirus Vaccine Efficacy and Effectiveness in Countries with High Child Mortality. Vaccines 2022, 10, 346. [Google Scholar] [CrossRef]
- Burnett, E.; Parashar, U.; Tate, J.E. Real-world effectiveness of rotavirus vaccines, 2006–2019: A literature review and meta-analysis. Lancet Glob. Health 2020, 8, 1195–1202. [Google Scholar] [CrossRef]
- Weintraub, E.S.; Baggs, J.; Duffy, J.; Vellozzi, C.; Belongia, E.A.; Irving, S.; Jackson, L.A. Risk of intussusception after monovalent rotavirus vaccination. N. Engl. J. Med. 2014, 370, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.B.; Macartney, K.K.; Lee, K.J.; Quinn, H.E.; Buttery, J.; Lopert, R.; Bines, J.; McIntyre, P.B. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin. Infect. Dis. 2013, 57, 1427–1434. [Google Scholar] [CrossRef]
- Patel, M.M.; Lopez-Collada, V.R.; Bulhoes, M.M.; De Oliveira, L.H.; Bautista Marquez, A.; Flannery, B. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N. Engl. J. Med. 2011, 364, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Stowe, J.; Andrews, N.; Ladhani, S.; Miller, E. The risk of intussusception following monovalent rotavirus vaccination in England: A self-controlled case- series evaluation. Vaccine 2016, 34, 3684–3689. [Google Scholar] [CrossRef]
- Kaplon, J.; Cros, G.; Ambert-Balay, K.; Leruez-Ville, M.; Chomton, M.; Fremy, C. Rotavirus vaccine virus shedding; viremia and clearance in infants with severe combined immune deficiency. Pediatr. Infect. Dis. 2015, 34, 326–328. [Google Scholar] [CrossRef]
- Klinkenberg, D.; Blohm, M.; Hoehne, M.; Mas Marques, A.; Malecki, M.; Schildgen, V. Risk of Rotavirus Vaccination for Children with SCID. Pediatr. Infect. Dis. 2015, 34, 114–115. [Google Scholar] [CrossRef]
- Palau, M.J.; Vescina, C.M.; Regairaz, L.; Cabanillas, D.; Stupka, J.A.; Degiuseppe, J.I. Persistent infection with a rotavirus vaccine strain in a child suffering from Severe Combined Immunodeficiency in Argentina. Rev. Argent. Microbiol. 2021, 53, 216–219. [Google Scholar] [CrossRef]
- Simsek, C.; Bloemen, M.; Jansen, D.; Descheemaeker, P.; Reynders, M.; Van Ranst, M.; Matthijnssens, J. Rotavirus vaccine-derived cases in Belgium: Evidence for reversion of attenuating mutations and alternative causes of gastroenteritis. Vaccine 2022, 35, 5114–5125. [Google Scholar] [CrossRef]
- Boom, J.A.; Sahni, L.C.; Payne, D.C.; Gautam, R.; Lyde, F.; Mijatovic- Rustempasic, S.; Bowen, M.D.; Tate, J.E.; Rench, M.A.; Gentsch, J.R.; et al. Symptomatic infection and detection of vaccine and vaccine- reassortant rotavirus strains in 5 children: A case series. J. Infect. Dis. 2012, 206, 1275–1279. [Google Scholar] [CrossRef]
- Bucardo, F.; Rippinger, C.M.; Svensson, L.; Patton, J.T. Vaccine-derived NSP2 segment in rotaviruses from vaccinated children with gastroenteritis in Nicaragua. Infect. Genet. Evol. 2012, 12, 1282–1294. [Google Scholar] [CrossRef]
- Dóró, R.; László, B.; Martella, V.; Leshem, E.; Gentsch, J.; Parashar, U.; Banyai, K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014, 28, 446–461. [Google Scholar] [CrossRef]
- Payne, D.C.; Edwards, K.M.; Bowen, M.D.; Keckley, E.; Peters, J.; Esona, M.D.; Teel, E.N.; Kent, D.; Parashar, U.D.; Gentsch, J.R. Sibling transmission of vaccine-derived rotavirus (RotaTeq) associated with rotavirus gastroenteritis. J. Pediatr. 2010, 125, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Lucien, M.A.B.; Esona, M.D.; Pierre, M.; Joseph, G.; Rivière, C.; Leshem, E.; Aliabadi, N.; Desormeaux, A.M.; Andre-Alboth, J.; Fitter, D.L.; et al. Diversity of rotavirus strains circulating in Haiti before and after introduction of monovalent vaccine. IJID Reg. 2022, 14, 146–151. [Google Scholar] [CrossRef]
- Hungerford, D.; Allen, D.J.; Nawaz, S.; Collins, S.; Ladhani, S.; Vivancos, R.; Iturriza-Gómara, M. Impact of rotavirus vaccination on rotavirus genotype distribution and diversity in England, September 2006 to August 2016. Euro Surveill. 2019, 24, 1700774. [Google Scholar] [CrossRef]
- Cates, J.E.; Amin, A.B.; Tate, J.E.; Lopman, B.; Parashar, U. Do Rotavirus Strains Affect Vaccine Effectiveness? A Systematic Review and Meta-analysis. J. Pediatr. Infect. Dis. 2021, 40, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.B.; Cates, J.E.; Liu, Z.; Wu, J.; Ali, I.; Rodriguez, A.; Panjwani, J.; Tate, J.E.; Lopman, B.A.; Parashar, U.D. Rotavirus genotypes in the post-vaccine era: A systematic review and meta-analysis of global, regional, and temporal trends in settings with and without rotavirus vaccine introduction. J. Infect. Dis. 2023, jiad403. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, C.D.; Ma, L.F.; Carey, M.E.; Steele, A.D. The rotavirus vaccine development pipeline. Vaccine 2019, 37, 7328–7335. [Google Scholar] [CrossRef]
- Song, J.M. Parenteral, non-live rotavirus vaccine: Recent history and future perspective. Clin. Exp. Vaccine Res. 2021, 10, 203–210. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Rahman, M.; Attoui, H.; Bányai, K.; Estes, M.K.; Gentsch, J.R.; Iturriza-Gómara, M.; Kirkwood, C.D.; Martella, V.; et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008, 153, 1621–1629. [Google Scholar] [CrossRef]
- Li, Y.; Xue, M.; Yu, L.; Luo, G.; Yang, H.; Jia, L.; Zeng, Y.; Li, T.; Ge, S.; Xia, N. Expression and characterization of a novel truncated rotavirus VP4 for the development of a recombinant rotavirus vaccine. Vaccine 2018, 36, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Khodabandehloo, M.; Shahrabadi, M.S.; Keyvani, H.; Bambai, B.; Sadigh, Z. Recombinant outer capsid glycoprotein (VP7) of rotavirus expressed in insect cells induces neutralizing antibodies in rabbit. Iran. J. Public Health 2012, 41, 73–84. [Google Scholar]
- Wen, X.; Cao, D.; Jones, R.W.; Li, J.; Szu, S.; Hoshino, Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine 2012, 30, 6121–6126. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Huang, P.; Jiang, X.; Tan, M. Immune response and protective efficacy of the S particle presented rotavirus VP8* vaccine in mice. Vaccine 2019, 37, 4103–4110. [Google Scholar] [CrossRef]
- Groome, M.J.; Koen, A.; Fix, A.; Page, N.; Jose, L.; Madhi, S.A.; McNeal, M.; Dally, L.; Cho, I.; Power, M.; et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: A randomised; double-blind; placebo-controlled trial. Lancet Infect. Dis. 2017, 17, 843–853. [Google Scholar] [CrossRef]
- Kondakova, O.A.; Ivanov, P.A.; Baranov, O.A.; Ryabchevskaya, E.M.; Arkhipenko, M.V.; Skurat, E.V.; Evtushenko, E.A.; Nikitin, N.A.; Karpova, O.V. Novel antigen panel for modern broad-spectrum recombinant rotavirus A vaccine. Clin. Exp. Vaccine Res. 2021, 10, 123–131. [Google Scholar] [CrossRef]
- Nair, N.; Feng, N.; Blum, L.K.; Sanyal, M.; Ding, S.; Jiang, B.; Sen, A.; Morton, J.M.; He, X.S.; Robinson, W.H.; et al. VP4-and VP7-specific antibodies mediate heterotypic immunity to rotavirus in humans. Sci. Transl. Med. 2017, 9, 5434. [Google Scholar] [CrossRef]
- Xue, M.; Yu, L.; Che, Y.; Lin, H.; Zeng, Y.; Fang, M.; Li, T.; Ge, S.; Xia, N. Characterization and protective efficacy in an animal model of a novel truncated rotavirus VP8 subunit parenteral vaccine candidate. Vaccine 2015, 33, 2606–2613. [Google Scholar] [CrossRef]
- Groome, M.J.; Fairlie, L.; Morrison, J.; Fix, A.; Koen, A.; Masenya, M.; Jose, L.; Shabir, M.; Page, N.; McNeal, M.; et al. Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine: A multisite; randomised; double-blind; placebo-controlled trial. Lancet Infect. Dis. 2020, 20, 851–863. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Mine, Y. Tandem copies of a human rotavirus VP8 epitope can induce specific neutralizing anti- bodies in BALB/c mice. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 1884–1893. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Yoo, D.; Mine, Y. Fine mapping of sequential neutralization epitopes on the subunit protein VP8 of human rotavirus. Biochem. J. 2003, 376, 269–275. [Google Scholar] [CrossRef]
- Sadiq, A.; Khan, J. Rotavirus in developing countries: Molecular diversity, epidemiological insights, and strategies for effective vaccination. Front. Microbiol. 2024, 5, 1297269. [Google Scholar] [CrossRef]
- Omatola, C.A.; Ogunsakin, R.E.; Olaniran, A.O. Prevalence, Pattern and Genetic Diversity of Rotaviruses among Children under 5 Years of Age with Acute Gastroenteritis in South Africa: A Systematic Review and Meta-Analysis. Viruses 2021, 13, 1905. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Zenin, V.A.; Nikitin, N.A.; Yurkova, M.S.; Ryabchevskaya, E.M.; Putlyaev, E.V.; Donchenko, E.K.; Kondakova, O.A.; Fedorov, A.N.; Atabekov, J.G.; et al. Study of rubella candidate vaccine based on a structurally modified plant virus. Antivir. Res. 2017, 144, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, A.O.; Ryabchevskaya, E.M.; Evtushenko, E.A.; Manukhova, T.I.; Kondakova, O.A.; Ivanov, P.A.; Arkhipenko, M.V.; Gushchin, V.A.; Nikitin, N.A.; Karpova, O.V. Vaccine Candidate Against COVID-19 Based on Structurally Modified Plant Virus as an Adjuvant. Front. Microbiol. 2022, 13, 845316. [Google Scholar] [CrossRef] [PubMed]
- Granovskiy, D.L.; Ryabchevskaya, E.M.; Evtushenko, E.A.; Kondakova, O.A.; Arkhipenko, M.V.; Kravchenko, T.B.; Bakhteeva, I.V.; Timofeev, V.S.; Nikitin, N.A.; Karpova, O.V. New formulation of a recombinant anthrax vaccine stabilised with structurally modified plant viruses. Front. Microbiol. 2022, 13, 1003969. [Google Scholar] [CrossRef] [PubMed]
- Bruckman, M.A.; Randolph, L.N.; VanMeter, A.; Hern, S.; Shoffstall, A.J.; Taurog, R.E.; Steinmetz, N.F. Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and -spheres in mice. Virology 2014, 449, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, N.A.; Zenin, V.A.; Trifonova, E.A.; Ryabchevskaya, E.M.; Kondakova, O.A.; Fedorov, A.N.; Atabekov, J.G.; Karpova, O.V. Assessment of structurally modified plant virus as a novel adjuvant in toxicity studies. Regul. Toxicol. Pharmacol. 2018, 97, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, N.A.; Malinin, A.S.; Rakhnyanskaya, A.A.; Trifonova, E.A.; Karpova, O.V.; Yaroslavov, A.A.; Atabekov, J.G. Use of a polycation spacer for noncovalent immobilization of albumin on thermally modified virus particles. Polym. Sci. Ser. A 2011, 53, 1026–1031. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Nikitin, N.A.; Kirpichnikov, M.P.; Karpova, O.V.; Atabekov, J.G. Obtaining and characterization of spherical particles—New biogenic platforms. Moscow Univ. Biol. Sci. Bull. 2015, 70, 194–197. [Google Scholar] [CrossRef]
- Society of Laboratory Animal Science. Specialist information from the Committee of Animal Welfare Officers (GV-SOLAS) and Working Group 4 in TVT Recommendation for Blood Sampling in Laboratory Animals, Especially Small Laboratory Animals. 2017. Available online: https://www.gv-solas.de/wp-content/uploads/2017/03/tie_blutentnahme17_e.pdf (accessed on 6 February 2024).
- Rotavirus Classification Working Group: RCWG. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 6 February 2024).
- João, E.D.; Munlela, B.; Chissaque, A.; Chilaúle, J.; Langa, J.; Augusto, O.; Boene, S.S.; Anapakala, E.; Sambo, J.; Guimarães, E.; et al. Molecular Epidemiology of Rotavirus A Strains Pre- and Post-Vaccine (Rotarix®) Introduction in Mozambique, 2012–2019: Emergence of Genotypes G3P[4] and G3P[8]. Pathogens 2020, 9, 671. [Google Scholar] [CrossRef]
- Knipping, K.; McNeal, M.M.; Crienen, A.; van Amerongen, G.; Garssen, J.; van’t Land, B. A gastrointestinal rotavirus infection mouse model for immune modulation studies. Virol. J. 2011, 8, 109. [Google Scholar] [CrossRef]
- Reimerink, J.H.J.; Boshuizen, J.A.; Einerhand, A.W.C.; Duizer, E.; van Amerongen, G.; Schmidt, N.; Koopmans, M.P.G. Systemic immune response after rotavirus inoculation of neonatal mice depends on source and level of purification of the virus: Implications for the use of heterologous vaccine candidates. J. Gen. Virol. 2007, 88, 604–612. [Google Scholar] [CrossRef]
- Rioux, G.; Babin, C.; Majeau, N.; Leclerc, D. Engineering of papaya mosaic virus (PapMV) nanoparticles through fusion of the HA11 peptide to several putative surface-exposed sites. PLoS ONE 2012, 7, 31925. [Google Scholar] [CrossRef]
- Koo, M.; Bendahmane, M.; Lettieri, G.A.; Paoletti, A.D.; Lane, T.E.; Fitchen, J.H.; Buchmeier, M.J.; Beachy, R.N. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc. Natl. Acad. Sci. USA 1999, 96, 7774–7779. [Google Scholar] [CrossRef]
- Shukla, S.; Wang, C.; Beiss, V.; Steinmetz, N.F. Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer. ACS Nano 2020, 14, 2994–3003. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granovskiy, D.L.; Khudainazarova, N.S.; Evtushenko, E.A.; Ryabchevskaya, E.M.; Kondakova, O.A.; Arkhipenko, M.V.; Kovrizhko, M.V.; Kolpakova, E.P.; Tverdokhlebova, T.I.; Nikitin, N.A.; et al. Novel Universal Recombinant Rotavirus A Vaccine Candidate: Evaluation of Immunological Properties. Viruses 2024, 16, 438. https://doi.org/10.3390/v16030438
Granovskiy DL, Khudainazarova NS, Evtushenko EA, Ryabchevskaya EM, Kondakova OA, Arkhipenko MV, Kovrizhko MV, Kolpakova EP, Tverdokhlebova TI, Nikitin NA, et al. Novel Universal Recombinant Rotavirus A Vaccine Candidate: Evaluation of Immunological Properties. Viruses. 2024; 16(3):438. https://doi.org/10.3390/v16030438
Chicago/Turabian StyleGranovskiy, Dmitriy L., Nelli S. Khudainazarova, Ekaterina A. Evtushenko, Ekaterina M. Ryabchevskaya, Olga A. Kondakova, Marina V. Arkhipenko, Marina V. Kovrizhko, Elena P. Kolpakova, Tatyana I. Tverdokhlebova, Nikolai A. Nikitin, and et al. 2024. "Novel Universal Recombinant Rotavirus A Vaccine Candidate: Evaluation of Immunological Properties" Viruses 16, no. 3: 438. https://doi.org/10.3390/v16030438