Immune and Metabolic Alterations in Children with Perinatal HIV Exposure
Abstract
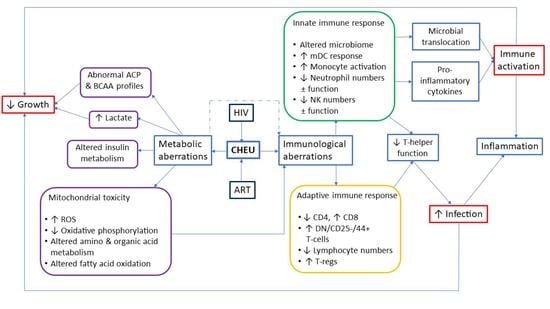
:1. Introduction
2. The global Picture of CHEU: Prevalence, Morbidity, and Mortality
3. Altered Innate Immune Responses Due to Peripartum HIV and/or ART Exposure
4. Altered Adaptive Immune Responses Due to Peripartum HIV and/or ART Exposure
5. Metabolomic Abnormalities of HIV-Exposed Uninfected Children
6. Implications for Infant Growth and Development
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joint United Nations Programme on HIV/AIDS. 2021 UNAIDS Global AIDS Update: Confronting inequalities—Lessons for Pandemic Responses from 40 Years of AIDS. Available online: https://www.unaids.org/en/resources/documents/2021/2021-global-aids-update (accessed on 26 February 2022).
- World Health Organization. WHO Validates Countries’ Elimination of Mother-to-Child Transmission of HIV and Syphilis. Available online: https://www.who.int/news/item/08-06-2016-who-validates-countries-elimination-of-mother-to-child-transmission-of-hiv-and-syphilis (accessed on 12 January 2023).
- Slogrove, A.L.; Johnson, L.F.; Powis, K.M. Population-level mortality associated with HIV exposure in HIV-uninfected infants in Botswana and South Africa: A model-based evaluation. J. Trop. Pediatr. 2019, 65, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Wedderburn, C.J.; Weldon, E.; Bertran-Cobo, C.; Rehman, A.M.; Stein, D.J.; Gibb, D.M.; Yeung, S.; Prendergast, A.J.; Donald, K.A. Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2022, 6, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Jones, C.E.; Prendergast, A.J. HIV-exposed, uninfected infants: New global challenges in the era of paediatric HIV elimination. Lancet Infect. Dis. 2016, 16, e92–e107. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Evans, S.J.; Orav, E.J.; Morris, V.; McIntosh, K.; Winter, H.S. Growth and body composition in children infected with the human immunodeficiency virus-1. Am. J. Clin. Nutr. 1993, 57, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Arpadi, S.M. Growth failure in children with HIV infection. J. Acquir. Immune Defic. Syndr. 2000, 25, S37–S42. [Google Scholar] [CrossRef]
- Isanaka, S.; Duggan, C.; Fawzi, W.W. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutr. Rev. 2009, 67, 343–359. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, K.K.; Lurie, M.N.; Triche, E.W.; De Bruyn, G.; Harwell, J.I.; McGarvey, S.T.; Gray, G.E. Growth of infants born to HIV-infected women in South Africa according to maternal and infant characteristics. Trop. Med. Int. Health 2010, 15, 1364–1374. [Google Scholar] [CrossRef]
- Voss, K.; Hong, H.S.; Bader, J.E.; Sugiura, A.; Lyssiotis, C.A.; Rathmell, J.C. A guide to interrogating immunometabolism. Nat. Rev. Immunol. 2021, 21, 637–652. [Google Scholar] [CrossRef]
- Slogrove, A.L.; Powis, K.M.; Johnson, L.F.; Stover, J.; Mahy, M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000–2018: A modelling study. Lancet Glob. Health 2020, 8, e67–e75. [Google Scholar] [CrossRef] [Green Version]
- Koyanagi, A.; Humphrey, J.H.; Ntozini, R.; Nathoo, K.; Moulton, L.H.; Iliff, P.; Mutasa, K.; Ruff, A.; Ward, B.; Group, Z.S. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr. Infect. Dis. J. 2011, 30, 45–51. [Google Scholar] [CrossRef]
- Adler, C.; Haelterman, E.; Barlow, P.; Marchant, A.; Levy, J.; Goetghebuer, T. Severe infections in HIV-exposed uninfected infants born in a European country. PloS ONE 2015, 10, e0135375. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.T.; Bonawitz, R.; Gill, C.J.; Thea, D.M.; Kleinman, M.; Useem, J.; Garrison, L.; Ceccarelli, R.; Udokwu, C.; Long, L. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS 2016, 30, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Labuda, S.M.; Huo, Y.; Kacanek, D.; Patel, K.; Huybrechts, K.; Jao, J.; Smith, C.; Hernandez-Diaz, S.; Scott, G.; Burchett, S. Rates of Hospitalization and Infection-Related Hospitalization Among Human Immunodeficiency Virus (HIV)–Exposed Uninfected Children Compared to HIV-Unexposed Uninfected Children in the United States, 2007–2016. Clin. Infect. Dis. 2020, 71, 332–339. [Google Scholar] [CrossRef]
- Slogrove, A.L.; Cotton, M.F.; Esser, M.M. Severe infections in HIV-exposed uninfected infants: Clinical evidence of immunodeficiency. J. Trop. Pediatr. 2010, 56, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slogrove, A.; Reikie, B.; Naidoo, S.; De Beer, C.; Ho, K.; Cotton, M.; Bettinger, J.; Speert, D.; Esser, M.; Kollmann, T. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J. Trop. Pediatr. 2012, 58, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Mussi-Pinhata, M.M.; Motta, F.; Freimanis-Hance, L.; de Souza, R.; Szyld, E.; Succi, R.C.; Christie, C.D.; Rolon, M.J.; Ceriotto, M.; Read, J.S. Lower respiratory tract infections among human immunodeficiency virus-exposed, uninfected infants. Int. J. Infect. Dis. 2010, 14, e176–e182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadnegahdar, R.; Fox, M.P.; Jeena, P.; Qazi, S.A.; Thea, D.M. Revisiting pneumonia and exposure status in infants born to HIV-infected mothers. Pediatr. Infect. Dis. J. 2014, 33, 70–72. [Google Scholar] [CrossRef] [Green Version]
- Marquez, C.; Okiring, J.; Chamie, G.; Ruel, T.D.; Achan, J.; Kakuru, A.; Kamya, M.R.; Charlebois, E.D.; Havlir, D.V.; Dorsey, G. Increased morbidity in early childhood among HIV-exposed uninfected children in Uganda is associated with breastfeeding duration. J. Trop. Pediatr. 2014, 60, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.S.; Wirth, K.E.; Steenhoff, A.P.; Cunningham, C.K.; Arscott-Mills, T.; Boiditswe, S.C.; Patel, M.Z.; Shah, S.S.; Finalle, R.; Makone, I. Treatment failures and excess mortality among HIV-exposed, uninfected children with pneumonia. J. Pediatr. Infect. Dis. Soc. 2015, 4, e117–e126. [Google Scholar] [CrossRef] [Green Version]
- Marinda, E.; Humphrey, J.H.; Iliff, P.J.; Mutasa, K.; Nathoo, K.J.; Piwoz, E.G.; Moulton, L.H.; Salama, P.; Ward, B.J.; Group, Z.S. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr. Infect. Dis. J. 2007, 26, 519–526. [Google Scholar] [CrossRef]
- Kuhn, L.; Kasonde, P.; Sinkala, M.; Kankasa, C.; Semrau, K.; Scott, N.; Tsai, W.-Y.; Vermund, S.H.; Aldrovandi, G.M.; Thea, D.M. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin. Infect. Dis. 2005, 41, 1654–1661. [Google Scholar] [CrossRef] [Green Version]
- de Deus, N.; Moraleda, C.; Serna-Bolea, C.; Renom, M.; Menendez, C.; Naniche, D. Impact of elevated maternal HIV viral load at delivery on T-cell populations in HIV exposed uninfected infants in Mozambique. BMC Infect. Dis. 2015, 15, 37. [Google Scholar] [CrossRef] [Green Version]
- Cutland, C.L.; Schrag, S.J.; Zell, E.R.; Kuwanda, L.; Buchmann, E.; Velaphi, S.C.; Groome, M.J.; Adrian, P.V.; Madhi, S.A.; PoPS Trial Team. Maternal HIV infection and vertical transmission of pathogenic bacteria. Pediatrics 2012, 130, e581–e590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Bosch, R.J.; Hunter, D.J.; Fataki, M.R.; Msamanga, G.I.; Fawzi, W.W. Maternal disease stage and child undernutrition in relation to mortality among children born to HIV-infected women in Tanzania. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 46, 599–606. [Google Scholar] [CrossRef]
- Ásbjörnsdóttir, K.H.; Slyker, J.A.; Maleche-Obimbo, E.; Wamalwa, D.; Otieno, P.; Gichuhi, C.M.; John-Stewart, G. Breastfeeding is associated with decreased risk of hospitalization among HIV-exposed, uninfected Kenyan infants. J. Hum. Lact. 2016, 32, NP61–NP66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostol, A.C.; Jensen, K.D.; Beaudin, A.E. Training the fetal immune system through maternal inflammation—A layered hygiene hypothesis. Front. Immunol. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Afran, L.; Garcia Knight, M.; Nduati, E.; Urban, B.; Heyderman, R.; Rowland-Jones, S. HIV-exposed uninfected children: A growing population with a vulnerable immune system? Clin. Exp. Immunol. 2014, 176, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, D.; Garvis, S. The importance of health and wellbeing. Health Wellbeing Child. 2014, 3, 18. [Google Scholar]
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Venter, W.D.; Sokhela, S.; Simmons, B.; Moorhouse, M.; Fairlie, L.; Mashabane, N.; Serenata, C.; Akpomiemie, G.; Masenya, M.; Qavi, A. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): Week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV 2020, 7, e666–e676. [Google Scholar]
- Newborn, A. Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States The information in the brief version is excerpted directly from the full-text guidelines. The brief version is a compilation of the tables and boxed recommendations. Management 2021, 12, E31. [Google Scholar]
- Akoto, C.; Norris, S.A.; Hemelaar, J. Maternal HIV infection is associated with distinct systemic cytokine profiles throughout pregnancy in South African women. Sci. Rep. 2021, 11, 1–15. [Google Scholar]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohman-Payne, B.; Gabriel, B.; Park, S.; Wamalwa, D.; Maleche-Obimbo, E.; Farquhar, C.; Bosire, R.K.; John-Stewart, G. HIV-exposed uninfected infants: Elevated cord blood Interleukin 8 (IL-8) is significantly associated with maternal HIV infection and systemic IL-8 in a Kenyan cohort. Clin. Transl. Med. 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenyogbe, N.; Dimitriu, P.; Cho, P.; Ruck, C.; Fortuno, E.S.; Cai, B.; Alimenti, A.; Côté, H.C.; Maan, E.J.; Slogrove, A.L. Innate immune responses and gut microbiomes distinguish HIV-exposed from HIV-unexposed children in a population-specific manner. J. Immunol. 2020, 205, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Makhubele, T.G.; Steel, H.C.; Anderson, R.; Van Dyk, G.; Theron, A.J.; Rossouw, T.M. Systemic immune activation profiles of HIV-1 subtype C-infected children and their mothers. Mediat. Inflamm. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, M.; Gouvea, A.; Ono, E.; Succi, R.C.M.; Pahwa, S.; Moraes-Pinto, M.I. Immune development in HIV-exposed uninfected children born to HIV-infected women. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e30. [Google Scholar] [CrossRef] [Green Version]
- Bender, J.M.; Li, F.; Martelly, S.; Byrt, E.; Rouzier, V.; Leo, M.; Tobin, N.; Pannaraj, P.S.; Adisetiyo, H.; Rollie, A.; et al. Maternal HIV infection influences the microbiome of HIV-uninfected infants. Sci. Transl. Med. 2016, 8, 349ra100. [Google Scholar] [CrossRef] [Green Version]
- Dillon, S.; Lee, E.; Kotter, C.; Austin, G.; Gianella, S.; Siewe, B.; Smith, D.; Landay, A.; McManus, M.; Robertson, C. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol. 2016, 9, 24–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, C.L.; Frank, D.N.; Robertson, C.E.; Ir, D.; Kofonow, J.M.; Montlha, M.P.; Mutsaerts, E.A.; Nunes, M.C.; Madhi, S.A.; Ghosh, D. Evolution of the Gut Microbiome in HIV-Exposed Uninfected and Unexposed Infants during the First Year of Life. Mbio 2022, 13, e0122922. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Kollmann, T.R.; Marchant, A.; MacGillivray, D.M. The Immune System of HIV-Exposed Uninfected Infants. Front. Immunol. 2016, 7, 383. [Google Scholar] [CrossRef] [Green Version]
- Bunders, M.; Cortina-Borja, M.; Thorne, C.; Kuijpers, T.; Newell, M.L.; Giaquinto, C.; Rampon, O.; Giacomet, V.; De Rossi, A.; Grosch-Worner, I. Levels and patterns of neutrophil cell counts over the first 8 years of life in children of HIV-1-infected mothers. AIDS 2004, 18, 2009–2017. [Google Scholar]
- Reikie, B.A.; Adams, R.C.; Leligdowicz, A.; Ho, K.; Naidoo, S.; Rusk, C.E.; de Beer, C.; Preiser, W.; Cotton, M.F.; Speert, D.P. Altered innate immune development in HIV-exposed uninfected infants. J. Acquir. Immune Defic. Syndr. 2014, 66, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloupazoa Siawaya, A.C.; Mveang-Nzoghe, A.; Mvoundza Ndjindji, O.; Mintsa Ndong, A.; Essone, P.N.; Djoba Siawaya, J.F. cases of impaired Oxidative Burst in hiV-exposed Uninfected infants’ neutrophils—A Pilot study. Front. Immunol. 2017, 8, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloupazoa Siawaya, A.C.; Mvoundza Ndjindji, O.; Kuissi Kamgaing, E.; Mveang-Nzoghe, A.; Mbani Mpega, C.N.; Leboueny, M.; Kengue Boussougou, R.; Mintsa Ndong, A.; Essone, P.N.; Djoba Siawaya, J.F. Altered toll-like receptor-4 response to lipopolysaccharides in infants exposed to HIV-1 and its preventive therapy. Front. Immunol. 2018, 9, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musimbi, Z.D.; Rono, M.K.; Otieno, J.R.; Kibinge, N.; Ochola-Oyier, L.I.; de Villiers, E.P.; Nduati, E.W. Peripheral blood mononuclear cell transcriptomes reveal an over-representation of down-regulated genes associated with immunity in HIV-exposed uninfected infants. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; Mussi-Pinhata, M.M.; Weinberg, A.; Yu, Q.; Cohen, R.; Harris, D.R.; Bowman, E.; Gabriel, J.; Kulkarni, M.; Funderburg, N. HIV-exposed uninfected infants have increased inflammation and monocyte activation. AIDS 2019, 33, 845. [Google Scholar] [CrossRef] [PubMed]
- Slyker, J.A.; Lohman-Payne, B.L.; John-Stewart, G.; Dong, T.; Mbori-Ngacha, D.; Tapia, K.; Atzberger, A.; Taylor, S.; Rowland-Jones, S.; Blish, C.A. The impact of HIV-1 infection and exposure on natural killer (NK) cell phenotype in Kenyan infants during the first year of life. Front. Immunol. 2012, 3, 399. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Jalbert, E.; de Almeida, V.; Canniff, J.; Lenz, L.L.; Mussi-Pinhata, M.M.; Cohen, R.A.; Yu, Q.; Amaral, F.R.; Pinto, J. Altered natural killer cell function in HIV-exposed uninfected infants. Front. Immunol. 2017, 8, 470. [Google Scholar] [CrossRef] [Green Version]
- Siawaya, A.C.M.; Mveang-Nzoghe, A.; Mpega, C.N.M.; Leboueny, M.; Ndjindji, O.M.; Ndong, A.M.; Essone, P.N.; Siawaya, J.F.D. Increased platelets count in HIV-1 uninfected infants born from HIV-1 infected mothers. Hematol. Rep. 2019, 11, 7056. [Google Scholar]
- Mesquita, E.C.; Hottz, E.D.; Amancio, R.T.; Carneiro, A.B.; Palhinha, L.; Coelho, L.E.; Grinsztejn, B.; Zimmerman, G.A.; Rondina, M.T.; Weyrich, A.S. Persistent platelet activation and apoptosis in virologically suppressed HIV-infected individuals. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hottz, E.; Bozza, F.; Bozza, P. Platelets in immune response to virus and immunopathology of viral infections. Front. Med. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Papakonstantinou, V.; Detopoulou, P.; Fragopoulou, E.; Chini, M.; Lazanas, M.C.; Antonopoulou, S. The role of platelet-activating factor in chronic inflammation, immune activation, and comorbidities associated with HIV infection. AIDS Rev. 2015, 17, 191. [Google Scholar] [PubMed]
- Tsoupras, A.B.; Chini, M.; Mangafas, N.; Tsogas, N.; Stamatakis, G.; Tsantila, N.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; Demopoulos, C.A. Platelet-activating factor and its basic metabolic enzymes in blood of naive HIV-infected patients. Angiology 2012, 63, 343–352. [Google Scholar] [CrossRef]
- Madzime, M.; Rossouw, T.M.; Theron, A.J.; Anderson, R.; Steel, H.C. Interactions of HIV and antiretroviral therapy with neutrophils and platelets. Front. Immunol. 2021, 12, 634386. [Google Scholar] [CrossRef] [PubMed]
- Brito-Pérez, Y.; Camacho-Pacheco, R.T.; Plazola-Camacho, N.; Soriano-Becerril, D.; Coronado-Zarco, I.A.; Arreola-Ramírez, G.; González-Pérez, G.; Herrera-Salazar, A.; Flores-González, J.; Bermejo-Haro, M.Y. Impaired T helper cell responses in human immunodeficiency virus-exposed uninfected newborns. Immun. Inflamm. Dis. 2021, 9, 1541–1553. [Google Scholar] [CrossRef]
- Kolte, L. Thymic Function in HIV-Infection. Dan. Med. J. 2013, 60, B4622. [Google Scholar]
- Kolte, L.; Rosenfeldt, V.; Vang, L.; Jeppesen, D.; Karlsson, I.; Ryder, L.P.; Skogstrand, K.; Nielsen, S.D. Reduced thymic size but no evidence of impaired thymic function in uninfected children born to human immunodeficiency virus-infected mothers. Pediatr. Infect. Dis. J. 2011, 30, 325–330. [Google Scholar] [CrossRef]
- Clerici, M.; Saresella, M.; Colombo, F.; Fossati, S.; Sala, N.; Bricalli, D.; Villa, M.L.; Ferrante, P.; Dally, L.; Vigano’, A. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood J. Am. Soc. Hematol. 2000, 96, 3866–3871. [Google Scholar]
- Nielsen, S.D.; Jeppesen, D.L.; Kolte, L.; Clark, D.R.; Sørensen, T.U.; Dreves, A.-M.; Ersbøll, A.K.; Ryder, L.P.; Valerius, N.H.; Nielsen, J.O. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood J. Am. Soc. Hematol. 2001, 98, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, M.; Pessoa, S.D.; Ono, E.; Machado, D.M.; Salomao, R.; Succi, R.C.d.M.; Pahwa, S.; de Moraes-Pinto, M.I. Low CD4+ T-cell levels and B-cell apoptosis in vertically HIV-exposed noninfected children and adolescents. J. Trop. Pediatr. 2010, 56, 427–432. [Google Scholar] [CrossRef]
- Clerici, M.; Butto, S.; Lukwiya, M.; Saresella, M.; Declich, S.; Trabattoni, D.; Pastori, C.; Piconi, S.; Fracasso, C.; Fabiani, M. Immune activation in Africa is environmentally-driven and is associated with upregulation of CCR5. AIDS 2000, 14, 2083–2092. [Google Scholar] [CrossRef]
- Gabriel, B.; Medin, C.; Alves, J.; Nduati, R.; Bosire, R.K.; Wamalwa, D.; Farquhar, C.; John-Stewart, G.; Lohman-Payne, B.L. Analysis of the TCR Repertoire in HIV-Exposed but Uninfected Infants. Sci. Rep. 2019, 9, 11954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, M.; Sandalova, E.; Low, D.; Gehring, A.J.; Fieni, S.; Amadei, B.; Urbani, S.; Chong, Y.-S.; Guccione, E.; Bertoletti, A. Trained immunity in newborn infants of HBV-infected mothers. Nat. Commun. 2015, 6, 6588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chougnet, C.; Kovacs, A.; Baker, R.; Mueller, B.U.; Luban, N.L.; Liewehr, D.J.; Steinberg, S.M.; Thomas, E.K.; Shearer, G.M. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J. Infect. Dis. 2000, 181, 1590–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Knight, M.A.; Nduati, E.; Hassan, A.S.; Gambo, F.; Odera, D.; Etyang, T.J.; Hajj, N.J.; Berkley, J.A.; Urban, B.C.; Rowland-Jones, S.L. Altered memory T-cell responses to Bacillus Calmette-Guerin and Tetanus Toxoid vaccination and altered cytokine responses to polyclonal stimulation in HIV-exposed uninfected Kenyan infants. PloS ONE 2015, 10, e0143043. [Google Scholar] [CrossRef] [PubMed]
- Kidzeru, E.B.; Hesseling, A.C.; Passmore, J.-A.S.; Myer, L.; Gamieldien, H.; Tchakoute, C.T.; Gray, C.M.; Sodora, D.L.; Jaspan, H.B. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS 2014, 28, 1421. [Google Scholar] [CrossRef] [Green Version]
- Jalbert, E.; Williamson, K.M.; Kroehl, M.E.; Johnson, M.J.; Cutland, C.; Madhi, S.A.; Nunes, M.C.; Weinberg, A. HIV-exposed uninfected infants have increased regulatory T cells that correlate with decreased T cell function. Front. Immunol. 2019, 10, 595. [Google Scholar] [CrossRef] [Green Version]
- Balle, C.; Armistead, B.; Kiravu, A.; Song, X.; Happel, A.-U.; Hoffmann, A.A.; Kanaan, S.B.; Nelson, J.L.; Gray, C.M.; Jaspan, H.B. Factors influencing maternal microchimerism throughout infancy and its impact on infant T cell immunity. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Kakkar, F.; Lamarre, V.; Ducruet, T.; Boucher, M.; Valois, S.; Soudeyns, H.; Lapointe, N. Impact of maternal HIV-1 viremia on lymphocyte subsets among HIV-exposed uninfected infants: Protective mechanism or immunodeficiency. BMC Infect. Dis. 2014, 14, 236. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Ramos, M.; Manno, D.; Kapambwe, M.; Ndumba, I.; Musonda, K.G.; Bates, M.; Chibumbya, J.; Siame, J.; Monze, M.; Filteau, S. Reduced Poliovirus vaccine neutralising-antibody titres in infants with maternal HIV-exposure. Vaccine 2013, 31, 2042–2049. [Google Scholar] [CrossRef] [Green Version]
- Reikie, B.A.; Naidoo, S.; Ruck, C.E.; Slogrove, A.L.; De Beer, C.; La Grange, H.; Adams, R.C.; Ho, K.; Smolen, K.; Speert, D.P. Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. Clin. Vaccine Immunol. 2013, 20, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Afran, L.; Jambo, K.C.; Nedi, W.; Miles, D.J.; Kiran, A.; Banda, D.H.; Kamg’ona, R.; Tembo, D.; Pachnio, A.; Nastouli, E. Defective Monocyte Enzymatic Function and an Inhibitory Immune Phenotype in Human Immunodeficiency Virus-Exposed Uninfected African Infants in the Era of Antiretroviral Therapy. J. Infect. Dis. 2022, 226, 1243–1255. [Google Scholar] [CrossRef]
- Kasahara, T.M.; Hygino, J.; Blanco, B.; Xavier, L.; Araújo-Lima, C.F.; Guillermo, L.V.; Bittencourt, V.C.B.; Guimarães, V.; Andrade, A.F.; Bento, C.A. The impact of maternal anti-retroviral therapy on cytokine profile in the uninfected neonates. Hum. Immunol. 2013, 74, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Falconer, O.; Newell, M.-L.; Jones, C.E. The effect of human immunodeficiency virus and cytomegalovirus infection on infant responses to vaccines: A review. Front. Immunol. 2018, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Coutsoudis, A.; Moodley, D.; Trabattoni, D.; Mngqundaniso, N.; Shearer, G.M.; Clerici, M.; Coovadia, H.M.; Stein, Z. T-helper cell responses to HIV envelope peptides in cord blood: Protection against intrapartum and breast-feeding transmission. AIDS 2001, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Greiter, B.M.; Kahlert, C.R.; Eberhard, N.; Sultan-Beyer, L.; Berger, C.; Paioni, P. Lymphocyte Subsets in HIV-Exposed Uninfected Infants: The Impact of Neonatal Postexposure Prophylaxis With Zidovudine. Open Forum Infect. Dis. 2020, 7, ofaa108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetghebuer, T.; Rowland-Jones, S.L.; Kollmann, T.R. Editorial: Immune mechanisms underlying the increased morbidity and mortality of HIV-Exposed Uninfected (HEU) children. Front. Immunol. 2017, 8, 1060. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Shalova, I.N.; Biswas, S.K. Metabolic regulation of macrophage phenotype and function. Immunol. Rev. 2017, 280, 102–111. [Google Scholar] [CrossRef]
- Boothby, M.; Rickert, R.C. Metabolic regulation of the immune humoral response. Immunity 2017, 46, 743–755. [Google Scholar] [CrossRef] [Green Version]
- McComsey, G.A.; Kang, M.; Ross, A.C.; Lebrecht, D.; Livingston, E.; Melvin, A.; Hitti, J.; Cohn, S.E.; Walker, U.A.; The AIDS Clinical Trials Group. Increased mtDNA levels without change in mitochondrial enzymes in peripheral blood mononuclear cells of infants born to HIV-infected mothers on antiretroviral therapy. HIV Clin. Trials 2008, 9, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.; Leong, T.; Avery, A.; Castillo-Duran, M.; Bonilla, H.; Lebrecht, D.; Walker, U.; Storer, N.; Labbato, D.; Khaitan, A. Effects of in utero antiretroviral exposure on mitochondrial DNA levels, mitochondrial function and oxidative stress. HIV Med. 2012, 13, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Côté, H.C.; Raboud, J.; Bitnun, A.; Alimenti, A.; Money, D.M.; Maan, E.; Costei, A.; Gadawski, I.; Diong, C.; Read, S. Perinatal exposure to antiretroviral therapy is associated with increased blood mitochondrial DNA levels and decreased mitochondrial gene expression in infants. J. Infect. Dis. 2008, 198, 851–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirmse, B.; Hobbs, C.V.; Peter, I.; LaPlante, B.; Caggana, M.; Kloke, K.; Raymond, K.; Summar, M.; Borkowsky, W. Abnormal newborn screening data and acylcarnitines in HIV/ARV-exposed infants. Pediatr. Infect. Dis. J. 2013, 32, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q.M. Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Calvo, S.E.; Clauser, K.R.; Mootha, V.K. MitoCarta2. 0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016, 44, D1251–D1257. [Google Scholar] [CrossRef] [Green Version]
- Young, M.J. Off-target effects of drugs that disrupt human mitochondrial DNA maintenance. Front. Mol. Biosci. 2017, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Schank, M.; Zhao, J.; Moorman, J.P.; Yao, Z.Q. The impact of HIV-and ART-induced mitochondrial dysfunction in cellular senescence and aging. Cells 2021, 10, 174. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Jao, J.; Powis, K.M.; Kirmse, B.; Chunli, Y.; Fanny, E.; Nshom, E.; Abrams, E.J.; Sperling, R.S.; Leroith, D.; Geffner, M.E. Lower mitochondrial DNA and altered mitochondrial fuel metabolism in HIV-exposed uninfected infants in Cameroon. AIDS 2017, 31, 2475. [Google Scholar] [CrossRef]
- Jao, J.; Kirmse, B.; Yu, C.; Qiu, Y.; Powis, K.; Nshom, E.; Epie, F.; Tih, P.M.; Sperling, R.S.; Abrams, E.J. Lower preprandial insulin and altered fuel use in HIV/antiretroviral-exposed infants in Cameroon. J. Clin. Endocrinol. Metab. 2015, 100, 3260–3269. [Google Scholar] [CrossRef] [Green Version]
- Wedderburn, C.J.; Evans, C.; Yeung, S.; Gibb, D.M.; Donald, K.A.; Prendergast, A.J. Growth and neurodevelopment of HIV-exposed uninfected children: A conceptual framework. Curr. HIV/AIDS Rep. 2019, 16, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Desquiret-Dumas, V.; D’Ottavi, M.; Monnin, A.; Goudenège, D.; Méda, N.; Vizeneux, A.; Kankasa, C.; Tylleskar, T.; Bris, C.; Procaccio, V.; et al. Long-Term Persistence of Mitochondrial DNA Instability in HIV-Exposed Uninfected Children during and after Exposure to Antiretroviral Drugs and HIV. Biomedicines 2022, 10, 1786. [Google Scholar] [CrossRef] [PubMed]
- Eckard, A.R.; Kirk, S.E.; Hagood, N.L. Contemporary issues in pregnancy (and offspring) in the current HIV era. Curr. HIV/AIDS Rep. 2019, 16, 492–500. [Google Scholar] [CrossRef]
- Esterhuizen, K.; Van der Westhuizen, F.H.; Louw, R. Metabolomics of mitochondrial disease. Mitochondrion 2017, 35, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Alimenti, A.; Burdge, D.R.; Ogilvie, G.S.; Money, D.M.; Forbes, J.C. Lactic acidemia in human immunodeficiency virus-uninfected infants exposed to perinatal antiretroviral therapy. Pediatr. Infect. Dis. J. 2003, 22, 782–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirmse, B.; Yao, T.-J.; Hofherr, S.; Kacanek, D.; Williams, P.L.; Hobbs, C.V.; Hazra, R.; Borkowsky, W.; Van Dyke, R.B.; Summar, M. Acylcarnitine profiles in HIV-exposed, uninfected neonates in the United States. AIDS Res. Hum. Retrovir. 2016, 32, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Adler-Wailes, D.C.; Liu, H.; Ahmad, F.; Feng, N.; Londos, C.; Manganiello, V.; Yanovski, J.A. Effects of the human immunodeficiency virus-protease inhibitor, ritonavir, on basal and catecholamine-stimulated lipolysis. J. Clin. Endocrinol. Metab. 2005, 90, 3251–3261. [Google Scholar] [CrossRef] [Green Version]
- Richmond, S.R.; Carper, M.J.; Lei, X.; Zhang, S.; Yarasheski, K.E.; Ramanadham, S. HIV-protease inhibitors suppress skeletal muscle fatty acid oxidation by reducing CD36 and CPT1 fatty acid transporters. Biochim. Biophys. Acta 2010, 1801, 559–566. [Google Scholar] [CrossRef]
- Apostolova, N.; Blas-García, A.; Esplugues, J.V. Mitochondrial interference by anti-HIV drugs: Mechanisms beyond Pol-γ inhibition. Trends Pharmacol. Sci. 2011, 32, 715–725. [Google Scholar] [CrossRef]
- Cassol, E.; Misra, V.; Holman, A.; Kamat, A.; Morgello, S.; Gabuzda, D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect. Dis. 2013, 13, 203. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [Green Version]
- Moutloatse, G.P.; Bunders, M.J.; van Reenen, M.; Mason, S.; Kuijpers, T.W.; Engelke, U.F.H.; Wevers, R.A.; Reinecke, C.J. Metabolic risks at birth of neonates exposed in utero to HIV-antiretroviral therapy relative to unexposed neonates: An NMR metabolomics study of cord blood. Metabolomics 2016, 12, 175. [Google Scholar] [CrossRef]
- Stepien, K.M.; Heaton, R.; Rankin, S.; Murphy, A.; Bentley, J.; Sexton, D.; Hargreaves, I.P. Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J. Clin. Med. 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoeman, J.C.; Moutloatse, G.P.; Harms, A.C.; Vreeken, R.J.; Scherpbier, H.J.; Van Leeuwen, L.; Kuijpers, T.W.; Reinecke, C.J.; Berger, R.; Hankemeier, T. Fetal Metabolic Stress Disrupts Immune Homeostasis and Induces Proinflammatory Responses in Human Immunodeficiency Virus Type 1–and Combination Antiretroviral Therapy–Exposed Infants. J. Infect. Dis. 2017, 216, 436–446. [Google Scholar] [CrossRef] [Green Version]
- Von Mollendorf, C.; Von Gottberg, A.; Tempia, S.; Meiring, S.; De Gouveia, L.; Quan, V.; Lengana, S.; Avenant, T.; Du Plessis, N.; Eley, B. Increased risk for and mortality from invasive pneumococcal disease in HIV-exposed but uninfected infants aged < 1 year in South Africa, 2009–2013. Clin. Infect. Dis. 2015, 60, 1346–1356. [Google Scholar] [PubMed] [Green Version]
- Adler, M.; Behel, S.; Duncan, D.; Houston, J.; Kalou, M.; Lasry, A.; Shaffer, N.; Sands, A.; Young, P. Technical guidance update on quality assurance for HIV rapid diagnostic tests. In Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Madhi, S.A.; Nachman, S.; Violari, A.; Kim, S.; Cotton, M.F.; Bobat, R.; Jean-Philippe, P.; McSherry, G.; Mitchell, C. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N. Engl. J. Med. 2011, 365, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges-Almeida, E.; Milanez, H.; Vilela, M.M.S.; Cunha, F.G.; Abramczuk, B.M.; Reis-Alves, S.C.; Metze, K.; Lorand-Metze, I. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infect. Dis. 2011, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Ruck, C.; Reikie, B.A.; Marchant, A.; Kollmann, T.R.; Kakkar, F. Linking susceptibility to infectious diseases to immune system abnormalities among HIV-exposed uninfected infants. Front. Immunol. 2016, 7, 310. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
du Toit, L.D.V.; Prinsloo, A.; Steel, H.C.; Feucht, U.; Louw, R.; Rossouw, T.M. Immune and Metabolic Alterations in Children with Perinatal HIV Exposure. Viruses 2023, 15, 279. https://doi.org/10.3390/v15020279
du Toit LDV, Prinsloo A, Steel HC, Feucht U, Louw R, Rossouw TM. Immune and Metabolic Alterations in Children with Perinatal HIV Exposure. Viruses. 2023; 15(2):279. https://doi.org/10.3390/v15020279
Chicago/Turabian Styledu Toit, Louise D. V., Andrea Prinsloo, Helen C. Steel, Ute Feucht, Roan Louw, and Theresa M. Rossouw. 2023. "Immune and Metabolic Alterations in Children with Perinatal HIV Exposure" Viruses 15, no. 2: 279. https://doi.org/10.3390/v15020279