Thinning Intensity Affects Soil-Atmosphere Fluxes of Greenhouse Gases and Soil Nitrogen Mineralization in a Lowland Poplar Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plantation Establishment and Experimental Design
2.3. Measurement of Soil Temperature, Moisture Content, and Water Table Level
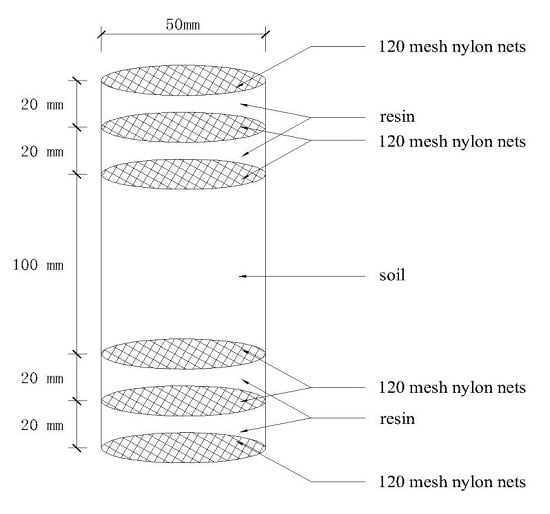
2.4. Soil N Mineralization and Sample Collection
2.5. Inorganic N Measurement and Data Calculation
2.6. Measurement of GHG Fluxes
2.7. Statistical Analysis
3. Results
3.1. Variation in Soil Environmental Factors
3.2. Variation in Fluxes of GHGs
3.3. Variation in Soil N Mineralization
4. Discussion
4.1. Effect of Thinning Regimes on C Sequestration and N Availability
4.2. Effect of Management Practices on GHG Emissions
4.3. Relationship of Environmental FactorstoGHG Emissions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saunders, M.; Tobin, B.; Black, K.; Gioria, M.; Nieuwenhuis, M.; Osborne, B.A. Thinning effects on the net ecosystem carbon exchange of a Sitka spruce forest are temperature-dependent. Agric. For. Meteorol. 2012, 157, 1–10. [Google Scholar] [CrossRef]
- Moreno-Fernández, D.; Díaz-Pinés, E.; Barbeito, I.; Sánchez-González, M.; Montes, F.; Rubio, A.; Caňllas, I. Temporal carbon dynamics over the rotation period of two alternative management systems in Mediterranean mountain Scots pine forests. For. Ecol. Manag. 2015, 348, 186–195. [Google Scholar] [CrossRef]
- Sun, S.Q.; Bhatti, J.S.; Jassal, R.S.; Chang, S.X.; Arevalo, C.M.; Black, T.A.; Sidders, D. Stand age and soil productivity control soil CO2 efflux and soil organic carbon dynamics in hybrid poplar plantations. Soil Sci. Soc. Am. J. 2015, 79, 1638–1649. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Price, D.T.; Cihlar, J.; Liu, J. Carbon offset potentials of four alternative forest management strategies in Canada: A simulation study. Mitig. Adapt. Strateg. Glob. Chang. 2000, 5, 143–169. [Google Scholar] [CrossRef]
- Adams, A.B.; Harrison, R.B.; Sletten, R.S.; Strahm, B.D.; Turnblom, E.C.; Jensen, C.M. Nitrogen-fertilization impacts on carbon sequestration and flux in managed coastal Douglas-fir stands of the Pacific Northwest. For. Ecol. Manag. 2005, 220, 313–325. [Google Scholar] [CrossRef]
- Liski, J.; Pussinen, A.; Pingoud, K.; Mäkipää, R.; Karjalainen, T. Which rotation length is favorable to carbon sequestration? Can. J. For. Res. 2001, 31, 2004–2013. [Google Scholar] [CrossRef]
- Minkkinen, K.; Laine, J.; Hökkä, H. Tree stand development and carbon sequestration in drained peatland stands in Finland—A simulation study. Silva Fenn. 2001, 35, 55–69. [Google Scholar] [CrossRef]
- Thornley, J.H.M.; Cannell, G.R. Managing forests for wood yield and carbon storage: A theoretical study. Tree Physiol. 2000, 20, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, P.; Strand, L.T. Thinning intensity effects on carbon and nitrogen stores and fluxes in a Norway spruce (Picea abies (L.) Karst.) stand after 33 years. For. Ecol. Manag. 2008, 256, 201–208. [Google Scholar] [CrossRef]
- Ruiz-Peinado, R.; Bravo-Oviedo, A.; Montero, G.; del Río, M. Carbon stocks in a Scots pine afforestation under different thinning intensities management. Mitig. Adapt. Strateg. Glob. Chang. 2014. [Google Scholar] [CrossRef]
- Johnson, D.W.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Harvest impacts on soil carbon storage in temperate forests. For. Ecol. Manag. 2010, 259, 857–866. [Google Scholar] [CrossRef]
- Fest, B.J.; Livesley, S.J.; Drösler, M.; van Gorsel, E.; Arndt, S.K. Soil–atmosphere greenhouse gas exchange in a cool, temperate Eucalyptus delegatensis forest in south-eastern Australia. Agric. For. Meteorol. 2009, 149, 393–406. [Google Scholar] [CrossRef]
- Arevalo, C.B.M.; Bhatti, J.S.; Chang, S.X.; Sidders, D. Land use change effects on ecosystem carbon balance: From agricultural to hybrid poplar plantation. Agric. Ecosyst. Environ. 2011, 141, 342–349. [Google Scholar] [CrossRef]
- Fang, S.Z.; Xue, J.H.; Tang, L.Z. Biomass production and carbon sequestration potential in poplar plantations with different management patterns. J. Environ. Manag. 2007, 85, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Z.; Li, H.Y.; Sun, Q.Q.; Chen, L.B. Biomass production and carbon stocks in poplar-crop intercropping systems: A case study in northwestern Jiangsu, China. Agrofor. Syst. 2010, 79, 213–222. [Google Scholar] [CrossRef]
- Pallardy, S.G.; Gibbins, D.E.; Rhoads, J.L. Biomass production by two-year-old poplar clones on floodplain sites in the Lower Midwest, USA. Agrofor. Syst. 2003, 59, 21–26. [Google Scholar] [CrossRef]
- Oelbermann, M.; Voroney, R.P.; Gordon, A.M. Carbon sequestration in tropical and temperate agroforestry systems: A review with examples from Costa Rica and southern Canada. Agric. Ecosyst. Environ. 2004, 104, 359–377. [Google Scholar] [CrossRef]
- Peichl, M.; Thevathasan, N.V.; Gordon, A.M.; Huss, J.; Abohassan, R.A. Carbon sequestration potentials in temperate tree-based intercropping systems, Southern Ontario, Canada. Agrofor. Syst. 2006, 66, 243–257. [Google Scholar] [CrossRef]
- International Poplar Commission (IPC). Improving Lives with Poplars and Willows: Synthesis of Country Progress Reports. In Proceedings of the IPC 24th Session, Dehradun, India, 30 October–2 November 2012.
- Verlinden, M.S.; Broeckx, L.S.; Van den Bulcke, J.; Van Acker, J.; Ceulemans, R. Comparative study of biomass determinants of 12 poplar (Populus) genotypes in a high-density short-rotation culture. For. Ecol. Manag. 2013, 307, 101–111. [Google Scholar] [CrossRef]
- Sabatti, M.; Fabbrini, F.; Harfouche, A.; Beritognolo, I.; Mareschi, L.; Carlini, M.; Paris, P.; Scarascia-Mugnozza, G. Evaluation of biomass production potential and heating value of hybrid poplar genotypes in a short-rotation culture in Italy. Ind. Crops Prod. 2014, 61, 62–73. [Google Scholar] [CrossRef]
- Devappa, R.K.; Rakshit, S.K.; Dekker, R.F.H. Forest biorefinery: Potential of poplar phytochemicals as value-added co-products. Biotechnol. Adv. 2015, 33, 681–716. [Google Scholar] [CrossRef] [PubMed]
- Archaux, F.; Chevalier, R.; Berthelot, A. Towards practices favourable to plant diversity in hybrid poplar plantations. For. Ecol. Manag. 2010, 259, 2410–2417. [Google Scholar] [CrossRef]
- Elferjani, R.; DesRochers, A.; Tremblay, F. Effects of mixing clones on hybrid poplar productivity, photosynthesis and root development in northeastern Canadian plantations. For. Ecol. Manag. 2014, 327, 157–166. [Google Scholar] [CrossRef]
- Henkel-Johnson, D.; Macdonald, S.E.; Bork, E.W.; Thomas, B.R. Influence of weed composition, abundance, and spatial proximity on growth in young hybrid poplar plantations. For. Ecol. Manag. 2016, 362, 55–68. [Google Scholar] [CrossRef]
- Fang, S.Z.; Xie, B.D.; Liu, J.J. Soil nutrient availability, poplar growth and biomass production on degraded agricultural soil under fresh grass mulch. For. Ecol. Manag. 2008, 255, 1802–1809. [Google Scholar] [CrossRef]
- Fang, S.Z.; Xie, B.D.; Liu, D.; Liu, J.J. Effects of mulching materials on nitrogen mineralization, nitrogen availability and poplar growth on degraded agricultural soil. New For. 2011, 41, 147–162. [Google Scholar] [CrossRef]
- Lafleur, B.; Thiffault, E.; Paré, D.; Camiré, C.; Bernier-Cardou, M.; Masse, S. Effects of hog manure application on the nutrition and growth of hybrid poplar (Populus spp.) and on soil solution chemistry in short-rotation woody crops. Agric. Ecosyst. Environ. 2012, 155, 95–104. [Google Scholar] [CrossRef]
- Liu, D.; Fang, S.Z.; Tian, Y.; Dun, X.J. Variation in rhizosphere soil microbial index of tree species on seasonal flooding land: An in situ rhizobox approach. Appl. Soil Ecol. 2012, 59, 1–11. [Google Scholar] [CrossRef]
- Yan, Y.F.; Fang, S.Z.; Tian, Y.; Deng, S.P.; Tang, L.Z.; Chuong, D.N. Influence of tree spacing on soil nitrogen mineralization and availability in hybrid poplar plantations. Forests 2015, 6, 636–649. [Google Scholar] [CrossRef]
- Wang, D.; Fan, J.Z.; Jing, P.P.; Cheng, Y.; Ruan, H.H. Analyzing the impact of climate and management factors on the productivity and soil carbon sequestration of poplar plantations. Environ. Res. 2016, 144, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Black, T.A.; Tan, C.S.; Nnyamah, J.U. Transpiration rate of Douglas fir trees in thinned and intact stands. Can. J. Soil Sci. 1980, 60, 625–631. [Google Scholar] [CrossRef]
- Hale, S. The effect of thinning intensity on the below-canopy light environment in a Sitka spruce plantation. For. Ecol. Manag. 2003, 179, 341–349. [Google Scholar] [CrossRef]
- Li, J.H.; Gu, M.M.; Meng, P.W.; Wang, Y.X.; Liu, Y.F.; Fang, S.Z. Atmosphere environment monitoring and data analysis in poplar plantation. Electr. Meas. Technol. 2014, 37, 117–120. (In Chinese) [Google Scholar]
- Armstrong, A.C. The measurement of watertable levels in structured clay soils by means of open auger hole. Earth Surf. Process. Landf. 1983, 8, 183–187. [Google Scholar] [CrossRef]
- Binkley, D.; Aber, J.; Pastor, J.; Nadelhoffer, K. Nitrogen availability in some Wisconsin forests: Comparisons of resin bags and on-site incubations. Biol. Fertil. Soils 1986, 2, 77–82. [Google Scholar] [CrossRef]
- Shibata, H.; Urakawa, R.; Toda, H.; Inagaki, Y.; Tateno, R.; Koba, K.; Nakanishi, A.; Fukuzawa, K.; Yamasaki, A. Changes in nitrogen transformation in forest soil representing the climate gradient of the Japanese archipelago. J. For. Res. 2011, 16, 374–385. [Google Scholar] [CrossRef]
- Gulledge, J.; Schimel, J.P. Controls on soil carbon dioxide and methane fluxes in a variety of Taiga forest stands in interior Alaska. Ecosystems 2000, 3, 269–282. [Google Scholar] [CrossRef]
- Chen, Q.F.; Ma, J.J.; Liu, J.H.; Zhao, C.S.; Liu, W. Characteristics of greenhouse gas emission in the Yellow River Delta wetland. Int. Biodeterior. Biodegrad. 2013, 85, 646–651. [Google Scholar] [CrossRef]
- Makinen, H.; Isomaki, A. Thinning intensity and long-term changes in increment and stem form of Scots pine trees. For. Ecol. Manag. 2004, 203, 21–34. [Google Scholar] [CrossRef]
- Slodicak, M.; Novak, J.; Skovsgaard, J.P. Wood production, litter fall and humus accumulation in a Czech thinning experiment in Norway spruce (Picea abies (L.) Karst.). For. Ecol. Manag. 2005, 209, 157–166. [Google Scholar] [CrossRef]
- Dun, X.J.; Qu, H.F.; Tian, Y.; Fang, S.Z.; Xu, X.Z. Effects of thinning treatments on soil available nitrogen of the poplar plantations in flooding land of Yangtze River. J. Nanjing For. Univ. 2013, 37, 45–50. (In Chinese) [Google Scholar]
- Tan, X.; Chang, S.X.; Comeau, P.G.; Wang, Y.H. Thinning effects on microbial biomass, N mineralization, and tree growth in a mid-rotation fire-origin lodgepole pine stand in the Lower Foothills of Alberta, Canada. For. Sci. 2008, 54, 465–474. [Google Scholar]
- Parmar, K.; Keith, A.M.; Rowe, R.L.; Sohi, S.P.; Moeckel, C.; Pereira, M.G.; McNamara, N.P. Bioenergy driven land use change impacts on soil greenhouse gas regulation under Short Rotation Forestry. Biomass Bioenerg. 2015, 82, 40–48. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.F.; Chang, S.X.; Qin, H.; Fu, S.L.; Jiang, P.K. Understory management and fertilization affected soil greenhouse gas emissions and labile organic carbon pools in a Chinese chestnut plantation. For. Ecol. Manag. 2015, 337, 126–134. [Google Scholar] [CrossRef]
- Koga, N.; Sawamoto, T.; Tsuruta, H. Life cycle inventory-based analysis of greenhouse gas emissions from arable land farming systems in Hokkaido, northern Japan. Soil Sci. Plant Nutr. 2006, 52, 564–574. [Google Scholar] [CrossRef]
- Berglund, Ö.; Berglund, K. Influence of water table level and soil properties on emissions of greenhouse gases from cultivated peat soil. Soil Biol. Biochem. 2011, 43, 923–931. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Papen, H. Four years continuous record of CH4-exchange between the atmosphere and untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany. Plant Soil 2002, 240, 77–90. [Google Scholar] [CrossRef]
- Merino, A.; Perez-Batallon, P.; Macias, F. Responses of soil organic matter and greenhouse gas fluxes to soil management and land use changes in a humid temperate region of southern Europe. Soil Biol. Biochem. 2004, 36, 917–925. [Google Scholar] [CrossRef]
- Price, S.J.; Sherlock, R.R.; Kelliher, F.M.; McSeveny, T.M.; Tate, K.R.; Condron, L.M. Pristine New Zealand forest soil is a strong methane sink. Glob. Chang. Biol. 2004, 10, 16–26. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chen, G.C.; Ye, Y. Coastal vegetation invasion increases greenhouse gas emission from wetland soils but also increases soil carbon accumulation. Sci. Total Environ. 2015, 526, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Tang, Y.H.; Hu, Q.W.; Hirata, S.; Tomomichi, K.; Mo, H.W.; Cao, G.M.; Mariko, S. Methane emissions from different vegetation zones in a Qinghai–Tibetan Plateau wetland. Soil Biol. Biochem. 2004, 36, 737–748. [Google Scholar] [CrossRef]
- Koelbener, A.; Strom, L.; Edwards, P.J.; Venterink, H.O. Plant species from mesotrophic wetlands cause relatively high methane emissions from peat soil. Plant Soil 2010, 326, 147–158. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 2012, 48, 175–181. [Google Scholar] [CrossRef]
- Wang, Y.K.; Fang, S.Z.; Chang, S.X.; Tian, Y. Non-additive effects of litter-mixing on soil carbon dioxide efflux from poplar-based agroforestry systems in the warm temperate region of China. Agrofor. Syst. 2014, 88, 193–203. [Google Scholar] [CrossRef]
- Berger, T.W.; Inselsbacher, E.; Zechmeister-Boltenstern, S. Carbon dioxide emissions of soils under pure and mixed stands of beech and spruce, affected by decomposing foliage litter mixtures. Soil Biol. Biochem. 2010, 42, 986–997. [Google Scholar] [CrossRef]
- Wessolek, G.; Schwärzel, K.; Renger, M.; Sauerbrey, R.; Siewert, C. Soil hydrology and CO2 release of peat soils. J. Plant Nutr. Soil Sci. 2002, 165, 494–500. [Google Scholar] [CrossRef]
- Kechavarzi, C.; Dawson, Q.; Leeds-Harrison, P.B.; Szatylowicz, J.; Gnatowski, T. Water-table management in lowland UK peat soils and its potential impact on CO2 emission. Soil Use Manag. 2007, 23, 359–367. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2001: The scientific basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]









| Soil Environmental Factor | Soil Greenhouse Gas Efflux (n = 48) | Net Soil N Mineralization (n = 16) | ||
|---|---|---|---|---|
| CO2 | CH4 | N2O | ||
| Temperature at 5 cm depth | 0.74 ** | 0.40 ** | 0.59 ** | 0.56 * |
| Temperature at 15 cm depth | 0.70 ** | 0.30 * | 0.64 ** | 0.49 * |
| Moisture at 5 cm depth | 0.15 | 0.76 ** | −0.18 | 0.65 ** |
| Moisture at 15 cm depth | 0.23 | 0.68 ** | −0.19 | 0.72 ** |
| Water table | −0.74 ** | −0.52 ** | −0.35 * | / |
| Thinning Treatment * | CO2 (kg·m−2·year−1) | CH4 (mg·m−2·year−1) | N2O (mg·m−2·year−1) |
|---|---|---|---|
| CK | 1.04 ± 0.02 d | −90.64 ± 30.41 d | 389.52 ± 53.76 a |
| MB | 1.27 ± 0.08 c | 81.87 ± 34.84 c | 227.46 ± 52.07 b |
| HB | 1.43 ± 0.01 b | 465.55 ± 114.59 b | 148.36 ± 38.32 c |
| HI | 1.71 ± 0.02 a | 982.92 ± 216.43 a | 187.01 ± 11.12 b,c |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Lin, D.; Tian, Y.; Hong, S. Thinning Intensity Affects Soil-Atmosphere Fluxes of Greenhouse Gases and Soil Nitrogen Mineralization in a Lowland Poplar Plantation. Forests 2016, 7, 141. https://doi.org/10.3390/f7070141
Fang S, Lin D, Tian Y, Hong S. Thinning Intensity Affects Soil-Atmosphere Fluxes of Greenhouse Gases and Soil Nitrogen Mineralization in a Lowland Poplar Plantation. Forests. 2016; 7(7):141. https://doi.org/10.3390/f7070141
Chicago/Turabian StyleFang, Shengzuo, Da Lin, Ye Tian, and Senxian Hong. 2016. "Thinning Intensity Affects Soil-Atmosphere Fluxes of Greenhouse Gases and Soil Nitrogen Mineralization in a Lowland Poplar Plantation" Forests 7, no. 7: 141. https://doi.org/10.3390/f7070141