Leaf Angle as a Criterion for Optimizing Irrigation in Forest Nurseries: Impacts on Physiological Seedling Quality and Performance after Planting in Pots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
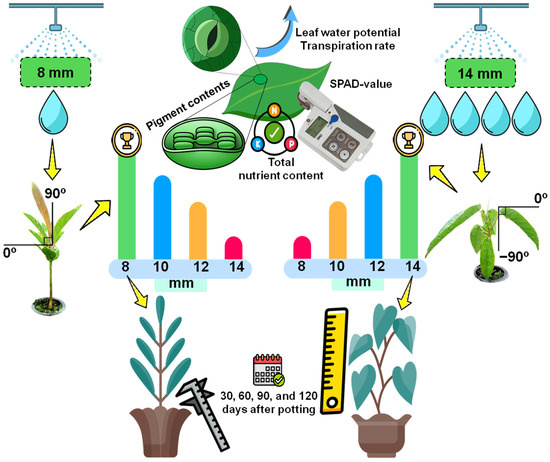
2.2. Leaf Angle Measurement and Experimental Treatments
2.3. Nursery Management
2.4. Physiological Analysis
2.4.1. Leaf Water Potential and Daily Transpiration Rate
2.4.2. Leaf Pigment Contents Analysis and SPAD Value
2.4.3. Total Nutrient Content Analysis
2.5. Seedling Growth Performance after Planting in Pot
2.6. Data Analysis
3. Results
3.1. Physiological Quality of Seedlings
3.2. Seedling Growth Performance after Planting in Pot
4. Discussion
4.1. Physiological Quality of Seedlings
4.2. Seedling Growth Performance after Planting in Pot
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haase, D.; Davis, A. Developing and Supporting Quality Nursery Facilities and Staff Are Necessary to Meet Global Forest and Landscape Restoration Needs. Reforesta 2017, 4, 69–93. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Kiiskila, S.B.; Haase, D.L. Seedling Ecophysiology—Five Questions To Explore in the Nursery for Optimizing Subsequent Field Success. Tree Plant. Notes 2020, 63, 112–127. [Google Scholar]
- Haase, D.L. Understanding Forest Seedling Quality: Measurements and Interpretation. Tree Plant. Notes 2008, 52, 24–30. [Google Scholar]
- Davis, A.S.; Jacobs, D.F. Quantifying Root System Quality of Nursery Seedlings and Relationship to Outplanting Performance. New For. 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Bertholdi, A.A.S.; Mantoan, L.P.B.; Vasconcellos, G.M.; Almeida, L.F.R. Photochemistry and Hydric Responses of Congeneric Croton Species at Restoration Sites under Dry Season: Implications for Species Selection. Theor. Exp. Plant Physiol. 2019, 31, 329–339. [Google Scholar] [CrossRef]
- da Silva, R.B.G.; Simões, D.; da Silva, M.R. Qualidade de Mudas Clonais de Eucalyptus Urophylla x E. Grandis Em Função Do Substrato. Rev. Bras. Eng. Agrícola Ambient. 2012, 16, 297–302. [Google Scholar] [CrossRef]
- da Silva, R.B.G.; Gabira, M.M.; Prado, D.Z.D.; Uesugi, G.; Simões, D.; da Silva, M.R. Influence of Mean Leaf Angles and Irrigation Volumes on Water Capture, Leaching, and Growth of Tropical Tree Seedlings. Forests 2020, 11, 1198. [Google Scholar] [CrossRef]
- Beeson, R.C.; Yeager, T.H. Plant Canopy Affects Sprinkler Irrigation Application Efficiency of Container-Grown Ornamentals. HortScience 2003, 38, 1373–1377. [Google Scholar] [CrossRef]
- Yeager, T.; Million, J.; Larsen, C.; Stamps, B. Florida Nursery Best Management Practices: Past, Present, and Future. Horttechnology 2010, 20, 82–88. [Google Scholar] [CrossRef]
- Reinhardt, D.; Kuhlemeier, C. Plant Architecture. EMBO Rep. 2002, 3, 846–851. [Google Scholar] [CrossRef]
- Liu, J.; Skidmore, A.K.; Wang, T.; Zhu, X.; Premier, J.; Heurich, M.; Beudert, B.; Jones, S. Variation of Leaf Angle Distribution Quantified by Terrestrial LiDAR in Natural European Beech Forest. ISPRS J. Photogramm. Remote Sens. 2019, 148, 208–220. [Google Scholar] [CrossRef]
- King, D.A. The Functional Significance of Leaf Angle in Eucalyptus. Aust. J. Bot. 1997, 45, 619. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf Size and Angle Vary Widely across Species: What Consequences for Light Interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- van Zanten, M.; Pons, T.L.; Janssen, J.A.M.; Voesenek, L.A.C.J.; Peeters, A.J.M. On the Relevance and Control of Leaf Angle. Crit. Rev. Plant Sci. 2010, 29, 300–316. [Google Scholar] [CrossRef]
- Hagemeier, M.; Leuschner, C. Functional Crown Architecture of Five Temperate Broadleaf Tree Species: Vertical Gradients in Leaf Morphology, Leaf Angle, and Leaf Area Density. Forests 2019, 10, 265. [Google Scholar] [CrossRef]
- Chianucci, F.; Pisek, J.; Raabe, K.; Marchino, L.; Ferrara, C.; Corona, P. A Dataset of Leaf Inclination Angles for Temperate and Boreal Broadleaf Woody Species. Ann. For. Sci. 2018, 75, 50. [Google Scholar] [CrossRef]
- Pisek, J.; Adamson, K. Dataset of Leaf Inclination Angles for 71 Different Eucalyptus Species. Data Br. 2020, 33, 106391. [Google Scholar] [CrossRef]
- Pisek, J.; Diaz-Pines, E.; Matteucci, G.; Noe, S.; Rebmann, C. On the Leaf Inclination Angle Distribution as a Plant Trait for the Most Abundant Broadleaf Tree Species in Europe. Agric. For. Meteorol. 2022, 323, 109030. [Google Scholar] [CrossRef]
- Niinemets, Ü. A Review of Light Interception in Plant Stands from Leaf to Canopy in Different Plant Functional Types and in Species with Varying Shade Tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Zou, J.; Zhong, P.; Hou, W.; Zuo, Y.; Leng, P. Estimating Needle and Shoot Inclination Angle Distributions and Projection Functions in Five Larix Principis-Rupprechtii Plots via Leveled Digital Camera Photography. Forests 2020, 12, 30. [Google Scholar] [CrossRef]
- Deguchi, R.; Koyama, K. Photosynthetic and Morphological Acclimation to High and Low Light Environments in Petasites Japonicus Subsp. Giganteus. Forests 2020, 11, 1365. [Google Scholar] [CrossRef]
- de Mattos, E.M.; Binkley, D.; Campoe, O.C.; Alvares, C.A.; Stape, J.L. Variation in Canopy Structure, Leaf Area, Light Interception and Light Use Efficiency among Eucalyptus Clones. For. Ecol. Manag. 2020, 463, 118038. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Vilagrosa, A.; Alonso-Forn, D.; Ferrio, J.P.; Sancho-Knapik, D.; Gil-Pelegrín, E. Living in Drylands: Functional Adaptations of Trees and Shrubs to Cope with High Temperatures and Water Scarcity. Forests 2020, 11, 1028. [Google Scholar] [CrossRef]
- Wu, X.; Fan, W.; Du, H.; Ge, H.; Huang, F.; Xu, X. Estimating Crown Structure Parameters of Moso Bamboo: Leaf Area and Leaf Angle Distribution. Forests 2019, 10, 686. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L.; Li, F. Dynamic Simulation of the Crown Net Photosynthetic Rate for Young Larix Olgensis Henry Trees. Forests 2019, 10, 321. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, Y.; Ying, H.; Wala, D.; Zhang, X.; Ruhan, A.; Rina, S.; Rina, S. Examining the Angular Effects of UAV-LS on Vegetation Metrics Using a Framework for Mediating Effects. Forests 2022, 13, 1221. [Google Scholar] [CrossRef]
- Wen, Y.; Zhuang, L.; Wang, H.; Hu, T.; Fan, W. An Automated Hemispherical Scanner for Monitoring the Leaf Area Index of Forest Canopies. Forests 2022, 13, 1355. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.; Yuan, X.; An, F.; Zhang, H.; Zhou, L.; Shi, J.; Yun, T. Simulating Wind Disturbances over Rubber Trees with Phenotypic Trait Analysis Using Terrestrial Laser Scanning. Forests 2022, 13, 1298. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, J.; Song, J.; Wang, J. Potential and Limits of Retrieving Conifer Leaf Area Index Using Smartphone-Based Method. Forests 2017, 8, 217. [Google Scholar] [CrossRef]
- Anna, K.-I.; Sylwia, Ł.; Marcin, Z.; Ewa, S.-O.; Wojtan, B. Variability of Leaf Wetting and Water Storage Capacity of Branches of 12 Deciduous Tree Species. Forests 2020, 11, 1158. [Google Scholar] [CrossRef]
- Dong, L.; Han, H.; Kang, F.; Cheng, X.; Zhao, J.; Song, X. Rainfall Partitioning in Chinese Pine (Pinus Tabuliformis Carr.) Stands at Three Different Ages. Forests 2020, 11, 243. [Google Scholar] [CrossRef]
- Fladung, M. Targeted CRISPR/Cas9-Based Knock-Out of the Rice Orthologs TILLER ANGLE CONTROL 1 (TAC1) in Poplar Induces Erect Leaf Habit and Shoot Growth. Forests 2021, 12, 1615. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Huang, Y.; Jia, Z.; Fang, Y. Leaf Venation Variation and Phenotypic Plasticity in Response to Environmental Heterogeneity in Parrotia Subaequalis (H. T. Chang) R. M. Hao et H. T. Wei, An Endemic and Endangered Tree Species from China. Forests 2018, 9, 247. [Google Scholar] [CrossRef]
- Roder, L.R.; Guerrini, I.A.; Lozano Sivisaca, D.C.; Yaguana Puglla, C.A.; Góes de Moraes, F.; Pinheiro da Silva, J.; Batista Fonseca, R.C.; Umbelino, M.T.; James, J.N.; Capra, G.F.; et al. Atlantic Rainforest Natural Regeneration in Fragmented Formations Affected by Increasing Human Disturbance. J. Environ. Manag. 2023, 325, 116521. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.E. Irrigation by Sprinkling; University of California: Berkeley, CA, USA, 1942. [Google Scholar]
- Merriam, J.L.; Keller, J. Farm Irrigation System Evaluation: A Guide for Management; Utah State University: Logan, UT, USA, 1978. [Google Scholar]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How Does Arbuscular Mycorrhizal Symbiosis Regulate Root Hydraulic Properties and Plasma Membrane Aquaporins in Phaseolus Vulgaris under Drought, Cold or Salinity Stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Landis, T.D. Mineral Nutrition as an Index of Seedling Quality: Principles and Applications. In Evaluating Seedling Quality: Principles, Procedures, and Predictive Abilities of Major Tests; Duryea, M.L., Ed.; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1985; pp. 29–48. [Google Scholar]
- Oliet, J.A.; Tejada, M.; Salifu, K.F.; Collazos, A.; Jacobs, D.F. Performance and Nutrient Dynamics of Holm Oak (Quercus Ilex L.) Seedlings in Relation to Nursery Nutrient Loading and Post-Transplant Fertility. Eur. J. For. Res. 2009, 128, 253–263. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Zhang, D.; Wei, H.; Guo, J. Using Morphological Attributes for the Fast Assessment of Nutritional Responses of Buddhist Pine (Podocarpus Macrophyllus [Thunb.] D. Don) Seedlings to Exponential Fertilization. PLoS ONE 2019, 14, e0225708. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods For Research Workers; Oliver and Boyd: London, UK, 1925. [Google Scholar]
- Scott, A.J.; Knott, M. A Cluster Analysis Method for Grouping Means in the Analysis of Variance. Biometrics 1974, 30, 507–512. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), version 8; StatSoft, Inc.: Tulsa, OK, USA, 2007. [Google Scholar]
- Delgado, L.G.M.; da Silva, R.B.G.; Gabira, M.M.; Rodrigues, A.L.; Simões, D.; de Almeida, L.F.R.; da Silva, M.R. Mean Leaf Angles Affect Irrigation Efficiency and Physiological Responses of Tropical Species Seedling. Forests 2022, 13, 832. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Why Seedlings Grow: Influence of Plant Attributes. New For. 2018, 49, 1–34. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; ArtMed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Yuan, Z.-S.; Liu, F.; Xie, B.-G.; Zhang, G.-F. The Growth-Promoting Effects of Endophytic Bacteria on Phyllostachys Edulis. Arch. Microbiol. 2018, 200, 921–927. [Google Scholar] [CrossRef]
- Yang, X.; Li, R.; Jablonski, A.; Stovall, A.; Kim, J.; Yi, K.; Ma, Y.; Beverly, D.; Phillips, R.; Novick, K.; et al. Leaf Angle as a Leaf and Canopy Trait: Rejuvenating Its Role in Ecology with New Technology. Ecol. Lett. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Durigan, G.; Baitello, J.B.; Franco, G.A.D.C.; Siqueira, M.F. Plantas Do Cerrado Paulista: Imagens de Uma Paisagem Ameaçada; Páginas & Letras: São Paulo, Brazil, 2004; ISBN 85-86508-32-2. [Google Scholar]
- Brandes, A.F.D.N.; Sánchez-Tapia, A.; Sansevero, J.B.B.; Albuquerque, R.P.; Barros, C.F. Fire Records in Tree Rings of Moquiniastrum Polymorphum: Potential for Reconstructing Fire History in the Brazilian Atlantic Forest. Acta Bot. Bras. 2019, 33, 61–66. [Google Scholar] [CrossRef]
- Morgan, J.M. Osmoregulation and Water Stress in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 299–319. [Google Scholar] [CrossRef]
- White, D.A.; Turner, N.C.; Galbraith, J.H. Leaf Water Relations and Stomatal Behavior of Four Allopatric Eucalyptus Species Planted in Mediterranean Southwestern Australia. Tree Physiol. 2000, 20, 1157–1165. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Biosynthesis, Accumulation and Emission of Carotenoids, α-Tocopherol, Plastoquinone, and Isoprene in Leaves under High Photosynthetic Irradiance. Photosynth. Res. 2007, 92, 163–179. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- de Sousa Leite, T.; da Silva Dias, N.; Oliveira de Freitas, R.M.; Dallabona Dombroski, J.L.; de Sousa Leite, M.; Martins de Farias, R. Ecophysiological and Biochemical Responses of Two Tree Species from a Tropical Dry Forest to Drought Stress and Recovery. J. Arid Environ. 2022, 200, 104720. [Google Scholar] [CrossRef]
- Pukacki, P.M.; Kamińska-Rożek, E. Effect of Drought Stress on Chlorophyll a Fluorescence and Electrical Admittance of Shoots in Norway Spruce Seedlings. Trees 2005, 19, 539–544. [Google Scholar] [CrossRef]
- Vasques, A.R.; Pinto, G.; Dias, M.C.; Correia, C.M.; Moutinho-Pereira, J.M.; Vallejo, V.R.; Santos, C.; Keizer, J.J. Physiological Response to Drought in Seedlings of Pistacia Lentiscus (Mastic Tree). New For. 2016, 47, 119–130. [Google Scholar] [CrossRef]
- Frosi, G.; Harand, W.; de Oliveira, M.T.; Pereira, S.; Cabral, S.P.; Montenegro, A.A.D.A.; Santos, M.G. Different Physiological Responses under Drought Stress Result in Different Recovery Abilities of Two Tropical Woody Evergreen Species. Acta Bot. Bras. 2017, 31, 153–160. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Kim, H.S. Responses to Drought Stress in Prunus Sargentii and Larix Kaempferi Seedlings Using Morphological and Physiological Parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Karimi, A.; Tabari, M.; Javanmard, Z.; Bader, M.K.-F. Drought Effects on Morpho-Physiological and Biochemical Traits in Persian Oak and Black Poplar Seedlings. Forests 2022, 13, 399. [Google Scholar] [CrossRef]
- Lassouane, N.; Aïd, F.; Lutts, S. Water Stress Impact on Young Seedling Growth of Acacia Arabica. Acta Physiol. Plant. 2013, 35, 2157–2169. [Google Scholar] [CrossRef]
- Firmo, W.C.A.; Miranda, M.V.; Coutinho, G.S.L.; Silveira, L.M.S.; Olea, R.S.G. Estudo Fitoquímico e Avaliação Da Atividade Antibacteriana de Lafoensia Pacari (Lythraceae). Publ. UEPG Cienc. Biol. Saude 2014, 20, 7–12. [Google Scholar] [CrossRef]
- Lima, P.C.; Santos, M.G.; Calabrese, K.S.; Silva, A.L.A.; Almeida, F. Avaliação Da Capacidade Leishmanicida de Espécies Vegetais Do Cerrado. Rev. Patol. Trop. 2015, 44, 45–55. [Google Scholar] [CrossRef]
- Bojovic, B.; Stojanovic, J. Chlorophyll and Carotenoid Content in Wheat Cultivars as a Function of Mineral Nutrition. Arch. Biol. Sci. 2005, 57, 283–290. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An Evaluation of Noninvasive Methods to Estimate Foliar Chlorophyll Content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Chenard, C.H.; Kopsell, D.A.; Kopsell, D.E. Nitrogen Concentration Affects Nutrient and Carotenoid Accumulation in Parsley. J. Plant Nutr. 2005, 28, 285–297. [Google Scholar] [CrossRef]
- Madeira, A.C.; Madeira, M.; Fabião, A.; Marques, P.; Carneiro, M. Avaliação Da Nutrição de Plantações Jovens de Eucalipto Por Análise Foliar e Métodos Não Destrutivos. Rev. Ciências Agrárias 2009, 32, 139–153. [Google Scholar] [CrossRef]
- Júnior, O.A.D.O.; Cairo, P.A.R.; De Novaes, A.B. Características Morfofisiológicas Associadas à Qualidade de Mudas de Eucalyptus Urophylla Produzidas Em Diferentes Substratos. Rev. Árvore 2011, 35, 1173–1180. [Google Scholar] [CrossRef]
- De Carvalho, R.P.; Davide, L.M.C.; Borges, F.L.G.; Davide, A.C.; Daniel, O. Respostas Morfofisiológicas Entre Procedências de Canafístula Submetidas a Diferentes Condições Hídricas e Nutricionais. Pesqui. Florest. Bras. 2015, 35, 179–188. [Google Scholar] [CrossRef]
- Uesugi, G.; Favan, J.R.; De Moraes, C.B.; Wanginiak, T.C.R.; da Silva, M.R. Utilização Do SPAD-502 Para a Predição Dos Teores de Nitrogênio Em Mudas de Croton Urucurana Baill. (Nota Científica). Rev. do Inst. Florest. 2015, 27, 177–181. [Google Scholar] [CrossRef]
- de Moura, A.R.; Nogueira, R.J.M.C.; da Silva, J.A.A.; de Lima, T.V. Relações Hídricas e Solutos Orgânicos Em Plantas Jovens de Jatropha Curcas L. Sob Diferentes Regimes Hídricos. Ciência Florest. 2016, 26, 345–354. [Google Scholar] [CrossRef]
- Acevedo, M.; Rubilar, R.; Dumroese, R.K.; Ovalle, J.F.; Sandoval, S.; Chassin-Trubert, R. Nitrogen Loading of Eucalyptus Globulus Seedlings: Nutritional Dynamics and Influence on Morphology and Root Growth Potential. New For. 2021, 52, 31–46. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bi, G.; Fuchigami, L.H.; Regan, R.P. Irrigation Frequency Alters Nutrient Uptake in Container-Grown Rhododendron Plants Grown with Different Rates of Nitrogen. HortScience 2012, 47, 189–197. [Google Scholar] [CrossRef]
- Silva, D.; Stuepp, C.A.; Wenfling, I.; Helm, C.; Angelo, A.C. Influence of Seed Storage Conditions on Quality of Torresea Acreana Seedlings. Cerne 2019, 25, 60–67. [Google Scholar] [CrossRef]
- Mangueira, J.R.S.A.; Holl, K.D.; Rodrigues, R.R. Enrichment Planting to Restore Degraded Tropical Forest Fragments in Brazil. Ecosyst. People 2019, 15, 3–10. [Google Scholar] [CrossRef]
- Simões, D.; Gil, J.F.S.; da Silva, R.B.G.; Munis, R.A.; da Silva, M.R. Stochastic Economic Analysis of Investment Projects in Forest Restoration Involving Containerized Tree Seedlings in Brazil. Forests 2021, 12, 1381. [Google Scholar] [CrossRef]
- Lopes, J.L.W.; Guerrini, I.A.; Saad, J.C.C.; da Silva, M.R. Nutrição Mineral de Mudas de Eucalipto Produzidas Sob Diferentes Lâminas de Irrigação e Substratos. Rev. Bras. Ciência Do Solo 2007, 31, 713–722. [Google Scholar] [CrossRef]
- Toca, A.; Oliet, J.A.; Villar-Salvador, P.; Martínez Catalán, R.A.; Jacobs, D.F. Ecologically Distinct Pine Species Show Differential Root Development after Outplanting in Response to Nursery Nutrient Cultivation. For. Ecol. Manag. 2019, 451, 117562. [Google Scholar] [CrossRef]
- Oliet, J.A.; Salazar, J.M.; Villar, R.; Robredo, E.; Valladares, F. Fall Fertilization of Holm Oak Affects N and P Dynamics, Root Growth Potential, and Post-Planting Phenology and Growth. Ann. For. Sci. 2011, 68, 647–656. [Google Scholar] [CrossRef]
- del Campo, A.D.; Segura-Orenga, G.; Molina, A.J.; González-Sanchis, M.; Reyna, S.; Hermoso, J.; Ceacero, C.J. On the Need to Further Refine Stock Quality Specifications to Improve Reforestation under Climatic Extremes. Forests 2022, 13, 168. [Google Scholar] [CrossRef]
- Vieira, D.L.M.; Rodrigues, S.B.; Jakovac, C.C.; da Rocha, G.P.E.; Reis, F.; Borges, A. Active Restoration Initiates High Quality Forest Succession in a Deforested Landscape in Amazonia. Forests 2021, 12, 1022. [Google Scholar] [CrossRef]
- Fiore, N.V.; Ferreira, C.C.; Dzedzej, M.; Massi, K.G. Monitoring of a Seedling Planting Restoration in a Permanent Preservation Area of the Southeast Atlantic Forest Biome, Brazil. Forests 2019, 10, 768. [Google Scholar] [CrossRef]
- Stape, J.L.; Gonçalves, J.L.M.; Gonçalves, A.N. Relationships between Nursery Practices and Field Performance for Eucalyptus Plantations in Brazil. New For. 2001, 22, 19–41. [Google Scholar] [CrossRef]
- Campbell, K.A.; Hawkins, C.D.B. Effect of Seed Source and Nursery Culture on Paper Birch (Betula Papyrifera) Uprooting Resistance and Field Performance. For. Ecol. Manag. 2004, 196, 425–433. [Google Scholar] [CrossRef]
- Olivo, V.B.; Buduba, C.G. Influencia de Seis Sustratos En El Crecimiento de Pinus Ponderosa Producido En Contenedores Bajo Condiciones de Invernáculo. Bosque (Valdivia) 2006, 27, 267–271. [Google Scholar] [CrossRef]
- Devetaković, J.R.; Pavlović, S.; Krinulović, L.; Janković, I.K. Field Performance of Austrian Pine Bareroot Seedlings in Comparision to Seedlings Pattern and Density in the Nursery. Reforesta 2021, 11, 27–35. [Google Scholar]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Relative Contribution of Initial Root and Shoot Morphology in Predicting Field Performance of Hardwood Seedlings. New For. 2005, 30, 235–251. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Davis, A.S.; Dumroese, R.K.; Burney, O.T. Nursery Cultural Techniques Facilitate Restoration of Acacia Koa Competing with Invasive Grass in a Dry Tropical Forest. Forests 2020, 11, 1124. [Google Scholar] [CrossRef]
- Grossnickle, S. Restoration Silviculture: An Ecophysiological Perspective—Lessons Learned across 40 Years. Reforesta 2016, 1, 1–36. [Google Scholar] [CrossRef]
- Thiffault, N.; Jobidon, R.; Munson, A.D. Comparing Large Containerized and Bareroot Conifer Stock on Sites of Contrasting Vegetation Composition in a Non-Herbicide Scenario. New For. 2014, 45, 875–891. [Google Scholar] [CrossRef]
- Gardiner, R.; Shoo, L.P.; Dwyer, J.M. Look to Seedling Heights, Rather than Functional Traits, to Explain Survival during Extreme Heat Stress in the Early Stages of Subtropical Rainforest Restoration. J. Appl. Ecol. 2019, 56, 2687–2697. [Google Scholar] [CrossRef]
- Johnson, D.M.; Smith, W.K. Refugial Forests of the Southern Appalachians: Photosynthesis and Survival in Current-Year Abies Fraseri Seedlings. Tree Physiol. 2005, 25, 1379–1387. [Google Scholar] [CrossRef]
- Pinto, J.; McNassar, B.; Kildisheva, O.; Davis, A. Stocktype and Vegetative Competition Influences on Pseudotsuga Menziesii and Larix Occidentalis Seedling Establishment. Forests 2018, 9, 228. [Google Scholar] [CrossRef]
- Gomes, J.M.; Couto, L.; Leite, H.G.; Xavier, A.; Garcia, S.L.R. Parâmetros Morfológicos Na Avaliação de Qualidade de Mudas de Eucalyptus Grandis. Rev. Árvore 2002, 26, 655–664. [Google Scholar] [CrossRef]
- de Souza, C.A.M.; de Oliveira, R.B.; Filho, S.M.; Lima, J.S.D.S. Crescimento Em Campo de Espécies Florestais Em Diferentes Condições de Adubações. Ciência Florest. 2006, 16, 243–249. [Google Scholar] [CrossRef]
- Downes, G.M.; Drew, D.; Battaglia, M.; Schulze, D. Measuring and Modelling Stem Growth and Wood Formation: An Overview. Dendrochronologia 2009, 27, 147–157. [Google Scholar] [CrossRef]
- Binotto, A.F.; Lúcio, A.D.C.; Lopes, S.J. Correlations between Growth Variables and the Dickson Quality Index in Forest Seedlings. Cerne 2010, 16, 457–464. [Google Scholar] [CrossRef]
- Speck, T.; Burgert, I. Plant Stems: Functional Design and Mechanics. Annu. Rev. Mater. Res. 2011, 41, 169–193. [Google Scholar] [CrossRef]
- South, D.B.; Rakestraw, J.L.; Lowerts, G.A. Early Gains from Planting Large-Diameter Seedlings and Intensive Management Are Additive for Loblolly Pine. New For. 2001, 22, 97–110. [Google Scholar] [CrossRef]
- Ivetić, V.; Grossnickle, S.; Škorić, M. Forecasting the Field Performance of Austrian Pine Seedlings Using Morphological Attributes. iFor. Biogeosci. For. 2016, 10, 99. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why Seedlings Survive: Influence of Plant Attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Grossnickle, S. Seedling Establishment on a Forest Restoration Site—An Ecophysiological Perspective. Reforesta 2018, 6, 110–139. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.B.G.; Simões, D.; Wendling, I.; do Prado, D.Z.; Sartori, M.M.P.; Bertholdi, A.A.d.S.; da Silva, M.R. Leaf Angle as a Criterion for Optimizing Irrigation in Forest Nurseries: Impacts on Physiological Seedling Quality and Performance after Planting in Pots. Forests 2023, 14, 1042. https://doi.org/10.3390/f14051042
da Silva RBG, Simões D, Wendling I, do Prado DZ, Sartori MMP, Bertholdi AAdS, da Silva MR. Leaf Angle as a Criterion for Optimizing Irrigation in Forest Nurseries: Impacts on Physiological Seedling Quality and Performance after Planting in Pots. Forests. 2023; 14(5):1042. https://doi.org/10.3390/f14051042
Chicago/Turabian Styleda Silva, Richardson Barbosa Gomes, Danilo Simões, Ivar Wendling, Débora Zanoni do Prado, Maria Márcia Pereira Sartori, Angelo Albano da Silva Bertholdi, and Magali Ribeiro da Silva. 2023. "Leaf Angle as a Criterion for Optimizing Irrigation in Forest Nurseries: Impacts on Physiological Seedling Quality and Performance after Planting in Pots" Forests 14, no. 5: 1042. https://doi.org/10.3390/f14051042