Identification of Alnus incana (L.) Moenx. × Alnus glutinosa (L.) Gaertn. Hybrids Using Metabolic Compounds as Chemotaxonomic Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objects and Material
2.2. Determination of Biologically Active Compounds in Wood Extracts
2.2.1. Extract Preparation
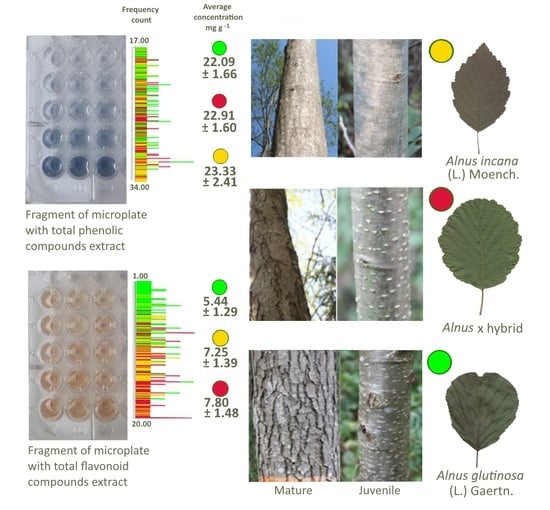
2.2.2. Determination of TPC Concentration
2.2.3. Determination of TFC Concentration
- TFC: y = 11.616x + 0.0634 (R2 = 0.9983);
- TPC: y = 5.5358x − 0.0423 (R2 = 0.9975).
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikola, P.; Uomala, P.; Mälkönen, E. Application of Biological Nitrogen Fixation in European Silviculture. In Biological Nitrogen Fixation in Forest Ecosystems: Foundations and Applications; Gordon, J.C., Wheeler, C.T., Eds.; Forestry Sciences; Springer: Dordrecht, The Netherlands, 1983; pp. 279–294. ISBN 978-94-009-6878-3. [Google Scholar]
- Claessens, H.; Oosterbaan, A.; Savill, P.; Rondeux, J. A Review of the Characteristics of Black Alder (Alnus glutinosa (L.) Gaertn.) and Their Implications for Silvicultural Practices. For. Int. J. For. Res. 2010, 83, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Durrant, T.H.; de Rigo, D.; Caudullo, G. Alnus incana in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-MiguelAyanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 24–25. [Google Scholar]
- Mandák, B.; Havrdová, A.; Krak, K.; Hadincová, V.; Vít, P.; Zákravský, P.; Douda, J. Recent Similarity in Distribution Ranges Does Not Mean a Similar Postglacial History: A Phylogeographical Study of the Boreal Tree Species Alnus incana Based on Microsatellite and Chloroplast DNA Variation. New Phytol. 2016, 210, 1395–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuvel, B.D.V. Alnus. In Wild Crop Relatives: Genomic and Breeding Resources–Forest Trees; Kolin, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–14. ISBN 978-3-642-21250-5. [Google Scholar]
- Ren, B.-Q.; Xiang, X.-G.; Chen, Z.-D. Species Identification of Alnus (Betulaceae) Using NrDNA and CpDNA Genetic Markers. Mol. Ecol. Resour. 2010, 10, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Jurkšienė, G.; Baliuckas, V. Natural Hybridization and Features between Alnus incana (L.) Moenc. and Alnus glutinosa (L.) Gaertn. in Lithuania. In Proceedings of the Agrosym 2019: X International Scientific Agriculture Symposium, Jahorina, East Sarajevo, Bosnia and Herzegovina, 3–6 October 2019; Kovacevic, D., Ed.; University of East Sarajevo: Faculty of Agriculture: East Sarajevo, Bosnia and Herzegovina, 2019. [Google Scholar]
- Jurkšienė, G.; Tamošaitis, S.; Kavaliauskas, D.; Buchovska, J.; Danusevičius, D.; Baliuckas, V. Identification of Alnus Glutinosa L. and A. incana (L.) Moench. Hybrids in Natural Forests Using Nuclear DNA Microsatellite and Morphometric Markers. Forests 2021, 12, 1504. [Google Scholar] [CrossRef]
- Altınyay, Ç.; Süntar, I.; Altun, L.; Keleş, H.; Küpeli Akkol, E. Phytochemical and Biological Studies on Alnus glutinosa subsp. Glutinosa, A. orientalis var. orientalis and A. orientalis var. Pubescens Leaves. J. Ethnopharmacol. 2016, 192, 148–160. [Google Scholar] [CrossRef]
- Saddique, M.; Kamran, M.; Shahbaz, M. Chapter 4—Differential Responses of Plants to Biotic Stress and the Role of Metabolites. In Plant Metabolites and Regulation Under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 69–87. ISBN 978-0-12-812689-9. [Google Scholar]
- Zidorn, C. Seasonal Variation of Natural Products in European Trees. Phytochem. Rev. 2018, 17, 923–935. [Google Scholar] [CrossRef]
- Skrypnik, L.; Grigorev, N.; Michailov, D.; Antipina, M.; Danilova, M.; Pungin, A. Comparative Study on Radical Scavenging Activity and Phenolic Compounds Content in Water Bark Extracts of Alder (Alnus glutinosa (L.) Gaertn.), Oak (Quercus Robur L.) and Pine (Pinus sylvestris L.). Eur. J. Wood Prod. 2019, 77, 879–890. [Google Scholar] [CrossRef]
- Oleksyn, J.; Karolewski, P.; Giertych, M.J.; Zytkowiak, R.; Reich, P.B.; Tjoelker, M.G. Primary and Secondary Host Plants Differ in Leaf-Level Photosynthetic Response to Herbivory: Evidence from Alnus and Betula Grazed by the Alder Beetle, Agelastica alni. New Phytol. 1998, 140, 239–249. [Google Scholar] [CrossRef]
- Peev, C.I.; Vlase, L.; Antal, D.S.; Dehelean, C.A.; Szabadai, Z. Determination of Some Polyphenolic Compounds in Buds of Alnus and Corylus Species by HPLC. Chem. Nat. Compd. 2007, 43, 259–262. [Google Scholar] [CrossRef]
- Vidaković, V.; Stefanović, M.; Novaković, M.; Jadranin, M.; Popović, Z.; Matić, R.; Tešević, V.; Bojović, S. Inter-and Intraspecific Variability of Selected Diarylheptanoid Compounds and Leaf Morphometric Traits in Alnus glutinosa and Alnus incana. Holzforschung 2018, 72, 1031–1041. [Google Scholar] [CrossRef]
- Vidaković, V.; Novaković, M.; Popović, Z.; Janković, M.; Matić, R.; Tešević, V.; Bojović, S. Significance of Diarylheptanoids for Chemotaxonomical Distinguishing between Alnus glutinosa and Alnus incana. Holzforschung 2018, 72, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Dahija, S.; Čakar, J.; Vidic, D.; Maksimović, M.; Parić, A. Total Phenolic and Flavonoid Contents, Antioxidant and Antimicrobial Activities of Alnus Glutinosa (L.) Gaertn., Alnus incana (L.) Moench and Alnus viridis (Chaix) DC. Extracts. Nat. Prod. Res. 2014, 28, 2317–2320. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.L.; Wallis, I.R.; Harwood, C.E.; Henson, M.; Foley, W.J. Heritable Variation in the Foliar Secondary Metabolite Sideroxylonal in Eucalyptus confers Cross-Resistance to Herbivores. Oecologia 2007, 153, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Külheim, C.; Yeoh, S.H.; Wallis, I.R.; Laffan, S.; Moran, G.F.; Foley, W.J. The Molecular Basis of Quantitative Variation in Foliar Secondary Metabolites in Eucalyptus globulus. New Phytol. 2011, 191, 1041–1053. [Google Scholar] [CrossRef]
- Ganthaler, A.; Stöggl, W.; Mayr, S.; Kranner, I.; Schüler, S.; Wischnitzki, E.; Sehr, E.M.; Fluch, S.; Trujillo-Moya, C. Association Genetics of Phenolic Needle Compounds in Norway Spruce with Variable Susceptibility to Needle Bladder Rust. Plant Mol. Biol. 2017, 94, 229–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Martínez, S.C.; Wheeler, N.C.; Ersoz, E.; Nelson, C.D.; Neale, D.B. Association Genetics in Pinus taeda L. I. Wood Property Traits. Genetics 2007, 175, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Dillon, S.K.; Nolan, M.; Li, W.; Bell, C.; Wu, H.X.; Southerton, S.G. Allelic Variation in Cell Wall Candidate Genes Affecting Solid Wood Properties in Natural Populations and Land Races of Pinus radiata. Genetics 2010, 185, 1477–1487. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.; Doerksen, T.; Boyle, B.; Clément, S.; Deslauriers, M.; Beauseigle, S.; Blais, S.; Poulin, P.-L.; Lenz, P.; Caron, S.; et al. Association Genetics of Wood Physical Traits in the Conifer White Spruce and Relationships with Gene Expression. Genetics 2011, 188, 197–214. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, J.W.; Resende Jr, M.F.R.; Munoz, P.; Walker, A.R.; Wegrzyn, J.L.; Nelson, C.D.; Neale, D.B.; Kirst, M.; Huber, D.A.; Gezan, S.A.; et al. Association Genetics of Oleoresin Flow in Loblolly Pine: Discovering Genes and Predicting Phenotype for Improved Resistance to Bark Beetles and Bioenergy Potential. New Phytol. 2013, 199, 89–100. [Google Scholar] [CrossRef]
- Lepoittevin, C.; Harvengt, L.; Plomion, C.; Garnier-Géré, P. Association Mapping for Growth, Straightness and Wood Chemistry Traits in the Pinus pinaster Aquitaine Breeding Population. Tree Genet. Genomes 2012, 8, 113–126. [Google Scholar] [CrossRef]
- Quesada, T.; Gopal, V.; Cumbie, W.P.; Eckert, A.J.; Wegrzyn, J.L.; Neale, D.B.; Goldfarb, B.; Huber, D.A.; Casella, G.; Davis, J.M. Association Mapping of Quantitative Disease Resistance in a Natural Population of Loblolly Pine (Pinus taeda L.). Genetics 2010, 186, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, P.C.; Pinno, B.D.; Šebesta, J.; Albrectsen, B.R.; Li, G.; Ivanova, N.; Kusbach, A.; Kuuluvainen, T.; Landhäusser, S.M.; Liu, H.; et al. A Global View of Aspen: Conservation Science for Widespread Keystone Systems. Glob. Ecol. Conserv. 2020, 21, e00828. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Sirgedaitė-Šėžienė, V.; Žemaitis, P.; Jansons, Ā.; Baliuckas, V. Resistance of Scots Pine Half-Sib Families to Heterobasidion Annosum in Progeny Field Trials. Silva Fenn. 2020, 54, 10276. [Google Scholar] [CrossRef]
- Vek, V.; Poljanšek, I.; Cerc Korošec, R.; Humar, M.; Oven, P. Impact of Steam-Sterilization and Oven Drying on the Thermal Stability of Phenolic Extractives from Pine and Black Locust Wood. J. Wood Chem. Technol. 2022, 42, 467–477. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Sirgedaitė-Šėžienė, V.; Žemaitis, P.; Baliuckas, V. The Resistance of Scots Pine (Pinus sylvestris L.) Half-Sib Families to Heterobasidion Annosum. Forests 2019, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Lauberts, M.; Pals, M. Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative. Plants 2021, 10, 2531. [Google Scholar] [CrossRef]
- Lithuania Climate: Average Temperature, Weather by Month, Lithuania Weather Averages—Climate-Data.Org. Available online: https://en.climate-data.org/europe/lithuania-51/ (accessed on 30 September 2022).
- Navasaitis, M.; Ozolinčius, R.; Smaliukas, D.; Balevičienė, J. Lietuvos Dendroflora: Monografija [Dendroflora of Lithuania: Monograph]; Lututė: Kaunas, Lithuania, 2003; ISBN 9955-575-35-2. [Google Scholar]
- Gange, A.C. Aphid Performance in An Alder (Alnus) Hybrid Zone. Ecology 1995, 76, 2074–2083. [Google Scholar] [CrossRef]
- Mejnartowicz, L. Morphology and Growth of Alnus incana × Glutinosa Hybrids. Arbor. Kórnickie Rocz. 1982, 26, 15–28. [Google Scholar]
- Banaev, E.; Bažant, V. Study of Natural Hybridization between Alnus incana (L.) Moench. and Alnus glutinosa (L.) Gaertn. J. For. Sci. 2007, 53, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Dróżdż, P.; Pyrzynska, K. Extracts from Pine and Oak Barks: Phenolics, Minerals and Antioxidant Potential. Int. J. Environ. Anal. Chem. 2021, 101, 464–472. [Google Scholar] [CrossRef]
- Kapustinskaitė, T. Juodalksnynai (Black alder forests). Vilnius Moksl. 1983, 117–124. (In Lithuanian) [Google Scholar]
- Li, X.; Wei, G.; El-Kassaby, Y.A.; Fang, Y. Hybridization and Introgression in Sympatric and Allopatric Populations of Four Oak Species. BMC Plant Biol. 2021, 21, 266. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, C.; Nawrot-Chorabik, K.; Woodward, S. Phenolic Compound Concentrations in Picea Abies Wood as an Indicator of Susceptibility towards Root Pathogens. For. Pathol. 2020, 50, e12652. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-Species versus Monocultures in Plantation Forestry: Development, Benefits, Ecosystem Services and Perspectives for the Future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Orians, C.M.; Griffiths, M.E.; Roche, B.M.; Fritz, R.S. Phenolic Glycosides and Condensed Tannins in Salix Sericea, S. Eriocephala and Their F1 Hybrids: Not All Hybrids Are Created Equal. Biochem. Syst. Ecol. 2000, 28, 619–632. [Google Scholar] [CrossRef]
- Hallgren, P.; Ikonen, A.; Hjältén, J.; Roininen, H. Inheritance Patterns of Phenolics in F1, F2, and Back-Cross Hybrids of Willows: Implications for Herbivore Responses to Hybrid Plants. J. Chem. Ecol. 2003, 29, 1143–1158. [Google Scholar] [CrossRef]
- Torp, M.; Lehrman, A.; Stenberg, J.A.; Julkunen-Tiitto, R.; Björkman, C. Performance of an Herbivorous Leaf Beetle (Phratora vulgatissima) on Salix F2 Hybrids: The Importance of Phenolics. J. Chem. Ecol. 2013, 39, 516–524. [Google Scholar] [CrossRef]
- Mihaylova-Kroumova, A.B.; Artiouchine, I.; Korenkov, V.D.; Wagner, G.J. Patterns of Inheritance of Acylsugar Acyl Groups in Selected Interspecific Hybrids of Genus Nicotiana. J. Plant Res. 2020, 133, 509–523. [Google Scholar] [CrossRef]
- Court, W.A.; Pocs, R.; Hendel, J.G.; Brandle, J.E. The Chemical Composition of Somatic Hybrids between Nicotiana tabacum and N. debneyi. Can. J. Plant Sci. 1992, 72, 209–215. [Google Scholar] [CrossRef]
- Altman, D.W.; Stipanovic, R.D.; Bell, A.A. Terpenoids in Foliar Pigment Glands of A, D, and AD Genome Cottons: Introgression Potential for Pest Resistance. J. Hered. 1990, 81, 447–454. [Google Scholar] [CrossRef]
- Haeggstroem, H.E. Variable Plant Quality and Performance of the Willow-Feeding Leaf Beetle Galerucella Lineola. Ph.D. Thesis, Swedish Univ. of Agricultural Sciences, Uppsala, Sweden, 1997. [Google Scholar]
- Morreel, K.; Goeminne, G.; Storme, V.; Sterck, L.; Ralph, J.; Coppieters, W.; Breyne, P.; Steenackers, M.; Georges, M.; Messens, E.; et al. Genetical Metabolomics of Flavonoid Biosynthesis in Populus: A Case Study. Plant J. 2006, 47, 224–237. [Google Scholar] [CrossRef] [PubMed]
- van Brederode, J.; van Wielink-Hillebrands, G.H.; van Nigtevecht, G. Dominance Relations between Isovitexin: 7-0-Glycosyltransferase Alleles in Melandrium. Mol. Genet. Genom. 1974, 130, 307–314. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics; Pearson Education India: Chennai, India, 1996. [Google Scholar]
- Cho, J.-S.; Nguyen, V.P.; Jeon, H.-W.; Kim, M.-H.; Eom, S.H.; Lim, Y.J.; Kim, W.-C.; Park, E.-J.; Choi, Y.-I.; Ko, J.-H. Overexpression of PtrMYB119, a R2R3-MYB Transcription Factor from Populus trichocarpa, Promotes Anthocyanin Production in Hybrid Poplar. Tree Physiol. 2016, 36, 1162–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boateng, K.; Hawkins, B.J.; Constabel, C.P.; Yanchuk, A.D.; Fellenberg, C. Red Alder Defense Mechanisms against Western Tent Caterpillar Defoliation. Can. J. For. Res. 2021, 51, 627–637. [Google Scholar] [CrossRef]
- Lučinskaitė, I.; Laužikė, K.; Žiauka, J.; Baliuckas, V.; Čėsna, V.; Sirgedaitė-Šėžienė, V. Assessment of Biologically Active Compounds, Organic Acids and Antioxidant Activity in Needle Extracts of Different Norway Spruce (Picea abies (L.) H. Karst) Half-Sib Families. Wood Sci. Technol. 2021, 55, 1221–1235. [Google Scholar] [CrossRef]
- Sirgedaitė-Šėžienė, V.; Lučinskaitė, I.; Mildažienė, V.; Ivankov, A.; Koga, K.; Shiratani, M.; Laužikė, K.; Baliuckas, V. Changes in Content of Bioactive Compounds and Antioxidant Activity Induced in Needles of Different Half-Sib Families of Norway Spruce (Picea abies (L.) H. Karst) by Seed Treatment with Cold Plasma. Antioxidants 2022, 11, 1558. [Google Scholar] [CrossRef]
- Miho, H.; Moral, J.; Barranco, D.; Ledesma-Escobar, C.A.; Priego-Capote, F.; Díez, C.M. Influence of Genetic and Interannual Factors on the Phenolic Profiles of Virgin Olive Oils. Food Chem. 2021, 342, 128357. [Google Scholar] [CrossRef]



| Forest Sites Information | 1 Site | 2 Site | 3 Site |
|---|---|---|---|
| Regional division | Biržai | Kretinga | Raseiniai |
| Forest enterprise | Latveliai | Vaineikiai | Birbiliškės |
| Forest block no. | 21 | 109 | 29 |
| Latitude | 56°21′23.51″ | 55°57′1.06″ | 55°13′6.39″ |
| Longitude | 24°50′19.39″ | 21°24′58.93″ | 23°13′38.00″ |
| Altitude, m | 54.0 | 53.6 | 81.0 |
| Forest type 1 | Lfs, cmh | Lcs, mox | Lfs, cmh |
| Zone (Köppen–Geiger index) 2 | Dfb | Cfb | Dfb |
| Annual average temperature (°C) | 7.5 | 8.2 | 7.9 |
| Average temperature in July (°C) | 19.1 | 18.8 | 19 |
| Average temperature in January (°C) | −3.8 | −1.9 | −3.4 |
| Precipitation (mm) | 748 | 770 | 733 |
| Average air humidity (%) | 76.1 | 77.3 | 77.2 |
| Biological Active Compounds | Age | Species * | No. | Average Mean, mg/g | ±Std. Deviation | Lower Bound on Mean (95%) | Upper Bound on Mean (95%) | One-Way ANOVA |
|---|---|---|---|---|---|---|---|---|
| TPC | Mature | AI | 45 | 22.43 a ** | 2.25 | 21.75 | 23.11 | F = 5.55 p = 0.0049 |
| AH | 45 | 21.99 ab | 1.32 | 21.59 | 22.39 | |||
| AG | 45 | 21.24 b | 1.38 | 20.83 | 21.66 | |||
| Juvenile | AI | 45 | 24.22 a | 2.23 | 23.55 | 24.89 | F = 6.61 p = 0.0018 | |
| AH | 45 | 23.82 a | 1.31 | 23.43 | 24.22 | |||
| AG | 45 | 22.43 b | 1.48 | 22.49 | 23.38 | |||
| Multivariate ANOVA | Species | F = 15.43 p < 0.0001 | ||||||
| Age | F = 91.61 p < 0.0001 | |||||||
| Location | F = 17.84 p < 0.0001 | |||||||
| Species × Age | F = 0.05 p = 0.9477 | |||||||
| Location × Age | F = 4.36 p = 0.138 | |||||||
| Species × Location | F = 2.37 p = 0.0538 | |||||||
| Species × Location × Age | F = 7.51 p < 0.0001 | |||||||
| TFC | Mature | AI | 45 | 7.75 a | 1.33 | 7.35 | 8.15 | F = 36.73 p < 0.0001 |
| AH | 45 | 7.82 a | 1.52 | 7.36 | 8.27 | |||
| AG | 45 | 5.56 b | 1.41 | 5.14 | 5.99 | |||
| Juvenile | AI | 45 | 6.75 b | 1.27 | 6.37 | 7.13 | F = 40.67 p < 0.0001 | |
| AH | 45 | 7.79 a | 1.46 | 7.35 | 8.23 | |||
| AG | 45 | 5.32 c | 1.16 | 4.97 | 5.67 | |||
| Multivariate ANOVA | Species | F = 88.28, p < 0.0001 | ||||||
| Age | F = 7.82, p = 0.0056 | |||||||
| Location | F = 12.25, p < 0.0001 | |||||||
| Species × Age | F = 3.81, p = 0.0235 | |||||||
| Location × Age | F = 6.93, p = 0.0012 | |||||||
| Species × Location | F = 1.38, p = 0.2404 | |||||||
| Species × Location × Age | F = 4.91, p = 0.0008 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkšienė, G.; Sirgedaitė-Šėžienė, V.; Juškauskaitė, A.; Baliuckas, V. Identification of Alnus incana (L.) Moenx. × Alnus glutinosa (L.) Gaertn. Hybrids Using Metabolic Compounds as Chemotaxonomic Markers. Forests 2023, 14, 150. https://doi.org/10.3390/f14010150
Jurkšienė G, Sirgedaitė-Šėžienė V, Juškauskaitė A, Baliuckas V. Identification of Alnus incana (L.) Moenx. × Alnus glutinosa (L.) Gaertn. Hybrids Using Metabolic Compounds as Chemotaxonomic Markers. Forests. 2023; 14(1):150. https://doi.org/10.3390/f14010150
Chicago/Turabian StyleJurkšienė, Girmantė, Vaida Sirgedaitė-Šėžienė, Aušra Juškauskaitė, and Virgilijus Baliuckas. 2023. "Identification of Alnus incana (L.) Moenx. × Alnus glutinosa (L.) Gaertn. Hybrids Using Metabolic Compounds as Chemotaxonomic Markers" Forests 14, no. 1: 150. https://doi.org/10.3390/f14010150